Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel pharmaceutical salts of cephalexin with organic counterions: structural analysis and properties
RSC Advances ( IF 3.9 ) Pub Date : 2022-12-06 , DOI: 10.1039/d2ra05565a Xiu-Ni Hua 1 , Xia Pan 1 , Yang Zhu 1, 2 , Zhuoer Cai 2 , Qi Song 1 , Yaozhenhui Li 1 , Wenbin Feng 1 , Xin Chen 1 , Hui Zhang 1 , Baiwang Sun 2
RSC Advances ( IF 3.9 ) Pub Date : 2022-12-06 , DOI: 10.1039/d2ra05565a Xiu-Ni Hua 1 , Xia Pan 1 , Yang Zhu 1, 2 , Zhuoer Cai 2 , Qi Song 1 , Yaozhenhui Li 1 , Wenbin Feng 1 , Xin Chen 1 , Hui Zhang 1 , Baiwang Sun 2
Affiliation
|
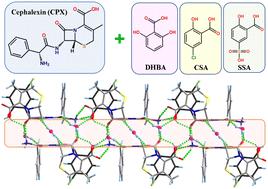
Three novel pharmaceutical salts of cephalexin (CPX) with 2,6-dihydroxybenzoic acid (DHBA), 5-chlorosalicylic acid (CSA) and 5-sulfosalicylic acid (SSA), which were obtained and thoroughly explored by various analytical techniques, were found to be crystallized invariably in hydrated forms. It is the proton transfer from carboxylic or sulfonic counterions to the CPX molecules that results in the salt formation. Crystal structure analyses reveal that the N–H⋯O and O–H⋯O hydrogen bonding interactions among the CPX, acidic guest molecules and water molecules play a crucial role in the packing motifs of crystal stabilization. All the salts exhibit higher solubility compared with the parent drug. These salts offer an alternative way of increasing the number of solid forms for CPX, which facilitates selection of a suitable form in the context of drug formulation development for further repurposing investigations.
中文翻译:

具有有机抗衡离子的头孢氨苄的新型药用盐:结构分析和性质
头孢氨苄 (CPX) 与 2,6-二羟基苯甲酸 (DHBA)、5-氯水杨酸 (CSA) 和 5-磺基水杨酸 (SSA) 的三种新型药用盐是通过各种分析技术获得和深入探索的,被发现可用于总是以水合形式结晶。正是质子从羧酸或磺酸抗衡离子转移到 CPX 分子,导致盐的形成。晶体结构分析表明,CPX、酸性客体分子和水分子之间的 N–H⋯O 和 O–H⋯O 氢键相互作用在晶体稳定的堆积基序中起着至关重要的作用。与母体药物相比,所有盐均表现出更高的溶解度。这些盐提供了一种增加 CPX 固体形式数量的替代方法,
更新日期:2022-12-06
中文翻译:

具有有机抗衡离子的头孢氨苄的新型药用盐:结构分析和性质
头孢氨苄 (CPX) 与 2,6-二羟基苯甲酸 (DHBA)、5-氯水杨酸 (CSA) 和 5-磺基水杨酸 (SSA) 的三种新型药用盐是通过各种分析技术获得和深入探索的,被发现可用于总是以水合形式结晶。正是质子从羧酸或磺酸抗衡离子转移到 CPX 分子,导致盐的形成。晶体结构分析表明,CPX、酸性客体分子和水分子之间的 N–H⋯O 和 O–H⋯O 氢键相互作用在晶体稳定的堆积基序中起着至关重要的作用。与母体药物相比,所有盐均表现出更高的溶解度。这些盐提供了一种增加 CPX 固体形式数量的替代方法,











































 京公网安备 11010802027423号
京公网安备 11010802027423号