Materials Today Communications ( IF 3.7 ) Pub Date : 2022-11-23 , DOI: 10.1016/j.mtcomm.2022.105013
Aneesha , Nobuhiro Ohta , Mohan Singh Mehata
|
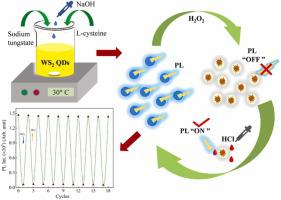
In most studies, liquid-exfoliated tungsten disulfide quantum dots (WS2 QDs) were synthesized at pH∼4 (acidic) and generally unstable. Therefore, a figment synthesis technique for creating stable WS2 QDs is essential. Here, functionalized blue fluorescent WS2 QDs were fabricated with a single-step hydrothermal growth method at pH∼11 without any subsequent modifications. Functional groups in the WS2 QDs give great stability, water dispersibility, and significant photoluminescence (PL) which depends on excitation wavelength. PL shows a multiexponential decay, probably as a result of the overlap of emissions from different emitting states. The absorption and PL of WS2 QDs were extremely sensitive to hydrogen peroxide (H2O2) in water. The absorption and PL of WS2 QDs quenched strongly and linearly with increasing H2O2 concentration from 0.33 nM to 594 µM. The quenching of both absorption and PL by H2O2 recovered nearly 100 % by adding 0.1 N HCl. The PL quenching/recovery showed cyclic stability for more than nine cycles, and the limit of detection (LoD) of H2O2 was estimated to be 1.7 × 10-6 M. The oxidation from W(IV) to W(VI) and subsequent reduction are considered to induce quenching of absorption/PL and recovery. PL decay profiles of WS2 QDs were also measured, and excited-state dynamics of the emitting state of WS2 QDs in the presence of H2O2 have been discussed. Thus, the fluorescent WS2 QDs having significant emission quantum yield can detect H2O2 with excellent cyclic stability and sensitivity in an aqueous environment, which will be further used for biological applications.
中文翻译:

用于传感 H2O2 的 WS2 QDs 的原位合成:吸收和光致发光的猝灭和恢复
在大多数研究中,液体剥离的二硫化钨量子点 (WS 2 QD) 是在 pH ~ 4(酸性)下合成的,通常不稳定。因此,用于创建稳定的 WS 2 QD 的虚构合成技术是必不可少的。在这里,功能化的蓝色荧光 WS 2 QD 是在 pH ~ 11 下采用一步水热生长方法制造的,没有任何后续修改。WS 2 QD中的官能团具有出色的稳定性、水分散性和显着的光致发光 (PL),这取决于激发波长。PL 显示多指数衰减,可能是由于不同发射状态的排放重叠。WS 2的吸收和PLQD 对水中的过氧化氢 (H 2 O 2 ) 极其敏感。随着 H 2 O 2浓度从 0.33 nM 增加到 594 µM ,WS 2 QD的吸收和 PL强烈地线性猝灭。通过添加 0.1 N HCl ,H 2 O 2对吸收和 PL 的淬灭几乎恢复了 100%。PL 淬灭/恢复显示循环稳定性超过 9 个循环,H 2 O 2的检测限 (LoD)估计为 1.7 × 10 -6 M。从 W(IV) 到 W(VI) 的氧化和随后的还原被认为会引起吸收/PL 和恢复的猝灭。WS 的 PL 衰减曲线还测量了2 QD,并且讨论了在存在 H 2 O 2的情况下 WS 2 QD 的发射态的激发态动力学。因此,具有显着发射量子产率的荧光WS 2 QDs可以在水环境中以优异的循环稳定性和灵敏度检测H 2 O 2,这将进一步用于生物学应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号