当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biodegradation of 2,5-Dihydroxypyridine by 2,5-Dihydroxypyridine Dioxygenase and Its Mutants: Insights into O–O Bond Activation and Flexible Reaction Mechanisms from QM/MM Simulations
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-12-05 , DOI: 10.1021/acs.inorgchem.2c03229 Yuzhuang Fu 1 , Binju Wang 1 , Zexing Cao 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-12-05 , DOI: 10.1021/acs.inorgchem.2c03229 Yuzhuang Fu 1 , Binju Wang 1 , Zexing Cao 1
Affiliation
|
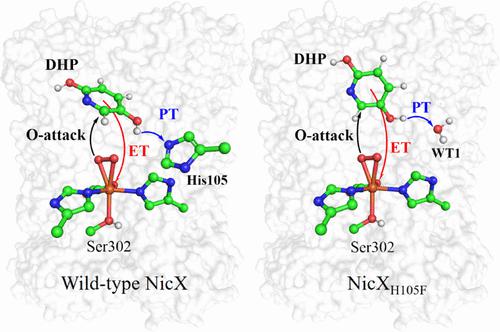
2,5-Dihydroxypyridine dioxygenase (NicX) from Pseudomonas putida KT2440 is a mononuclear non-heme iron oxygenase responsible for the biodegradation of 2,5-dihydroxypyridine (DHP) to N-formylmaleamic acid (NFM). Here, extensive quantum mechanical–molecular mechanical (QM/MM) calculations and molecular dynamics (MD) simulations are used to elucidate the degradation mechanism of DHP by wild-type NicX and its H105F variant (NicXH105F) and the roles of key residues. In particular, NicX and NicXH105F can catalyze the ring opening degradation of DHP to NFM, but flexible mechanisms are adopted therein. Both reactions of NicX and NicXH105F are initiated by the attack of FeIII superoxide species onto the substrate, during which a proton-coupled electron transfer (PCET) process is involved. For wild-type NicX, the PCET reaction is mediated by the adjacent His105, while the further proton transfer from His105 to the peroxo species can remarkably enhance the following O–O cleavage. However, for the NicXH105F mutant, a water molecule replaces the role of residue His105, which not only stabilizes the substrate binding via a H bonding network but also functions as a base to mediate the PCET process. For the NicXH105A mutant, MD simulations show that the disruption of the H bonding network can displace the substrate binding, leading to the loss of enzyme activity. These findings can expand our understanding of the PCET-mediated O–O bond activation and the flexible catalytic routes in various mutants, which have general implications on enzyme catalysis.
中文翻译:

2,5-二羟基吡啶双加氧酶及其突变体对 2,5-二羟基吡啶的生物降解:从 QM/MM 模拟深入了解 O-O 键激活和柔性反应机制
来自恶臭假单胞菌KT2440 的 2,5-二羟基吡啶双加氧酶 (NicX)是一种单核非血红素铁加氧酶,负责将 2,5-二羟基吡啶 (DHP) 生物降解为N-甲酰马来酰胺酸 (NFM)。在这里,广泛的量子力学-分子力学 (QM/MM) 计算和分子动力学 (MD) 模拟用于阐明野生型 NicX 及其 H105F 变体 (NicX H105F ) 对 DHP 的降解机制以及关键残基的作用。特别地,NicX和NicX H105F可以催化DHP开环降解为NFM,但其中采用了灵活的机制。NicX 和 NicX H105F的反应都是由 Fe III的攻击引发的超氧化物物种到基板上,在此期间涉及质子耦合电子转移(PCET)过程。对于野生型 NicX,PCET 反应由相邻的 His105 介导,而从 His105 到过氧物种的进一步质子转移可以显着增强随后的 O-O 裂解。然而,对于 NicX H105F突变体,水分子取代了残基 His105 的作用,它不仅通过 H 键合网络稳定底物结合,而且还充当介导 PCET 过程的碱基。对于 NicX H105A突变体,MD 模拟显示 H 键合网络的破坏可以取代底物结合,导致酶活性丧失。这些发现可以扩展我们对 PCET 介导的 O-O 键激活和各种突变体中灵活的催化途径的理解,这对酶催化具有普遍意义。
更新日期:2022-12-05
中文翻译:

2,5-二羟基吡啶双加氧酶及其突变体对 2,5-二羟基吡啶的生物降解:从 QM/MM 模拟深入了解 O-O 键激活和柔性反应机制
来自恶臭假单胞菌KT2440 的 2,5-二羟基吡啶双加氧酶 (NicX)是一种单核非血红素铁加氧酶,负责将 2,5-二羟基吡啶 (DHP) 生物降解为N-甲酰马来酰胺酸 (NFM)。在这里,广泛的量子力学-分子力学 (QM/MM) 计算和分子动力学 (MD) 模拟用于阐明野生型 NicX 及其 H105F 变体 (NicX H105F ) 对 DHP 的降解机制以及关键残基的作用。特别地,NicX和NicX H105F可以催化DHP开环降解为NFM,但其中采用了灵活的机制。NicX 和 NicX H105F的反应都是由 Fe III的攻击引发的超氧化物物种到基板上,在此期间涉及质子耦合电子转移(PCET)过程。对于野生型 NicX,PCET 反应由相邻的 His105 介导,而从 His105 到过氧物种的进一步质子转移可以显着增强随后的 O-O 裂解。然而,对于 NicX H105F突变体,水分子取代了残基 His105 的作用,它不仅通过 H 键合网络稳定底物结合,而且还充当介导 PCET 过程的碱基。对于 NicX H105A突变体,MD 模拟显示 H 键合网络的破坏可以取代底物结合,导致酶活性丧失。这些发现可以扩展我们对 PCET 介导的 O-O 键激活和各种突变体中灵活的催化途径的理解,这对酶催化具有普遍意义。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号