当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting the Steric Effect and Low Dielectric Constant of 1,2-Dimethoxypropane for 4.3 V Lithium Metal Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-12-04 , DOI: 10.1021/acsenergylett.2c02003 Eunseok Park 1 , Jongseok Park 1 , Kyunam Lee 1 , Yan Zhao 2 , Tianhong Zhou 2 , Gyuleen Park 1 , Min-Gi Jeong 3 , Minseok Choi 1 , Dong-Joo Yoo 4 , Hun-Gi Jung 3 , Ali Coskun 2 , Jang Wook Choi 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-12-04 , DOI: 10.1021/acsenergylett.2c02003 Eunseok Park 1 , Jongseok Park 1 , Kyunam Lee 1 , Yan Zhao 2 , Tianhong Zhou 2 , Gyuleen Park 1 , Min-Gi Jeong 3 , Minseok Choi 1 , Dong-Joo Yoo 4 , Hun-Gi Jung 3 , Ali Coskun 2 , Jang Wook Choi 1
Affiliation
|
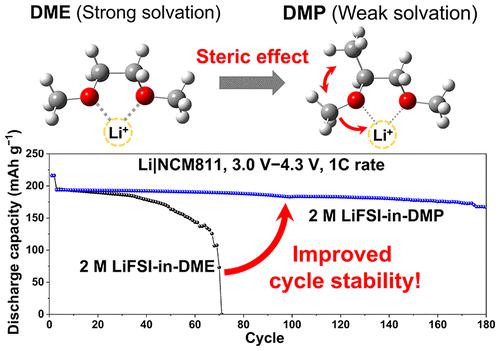
1,2-Dimethoxyethane (DME) has been widely used as an electrolyte solvent for lithium metal batteries on account of its intrinsic reductive stability; however, its low oxidative stability presents a major challenge for use in high-voltage Li metal batteries (LMBs). In this direction, herein, we introduce a new low-dielectric solvent, 1,2-dimethoxypropane (DMP), as an electrolyte solvent. Compared to DME, DMP has decreased solvation power owing to its increased steric effects, thus promoting anion–Li+ interactions. This controlled solvation structure of the 2 M LiFSI-in-DMP electrolyte facilitated the formation of an anion-driven, stable interface at the lithium metal anode and oxidative stability for compatibility with widely adopted cathodes to afford Li|LiFePO4 and Li|LiNi0.8Co0.1Mn0.1O2 cells with decent cycling stability. These results imply the usefulness of steric control as an alternative strategy to commonly used fluorination to fine-tune the solvation power and, in general, the design of new solvents for practical lithium metal batteries.
中文翻译:

利用 1,2-二甲氧基丙烷的空间效应和低介电常数制造 4.3 V 锂金属电池
1,2-二甲氧基乙烷 (DME) 因其固有的还原稳定性而被广泛用作锂金属电池的电解质溶剂;然而,其低氧化稳定性对在高压锂金属电池 (LMB) 中的应用提出了重大挑战。在这个方向上,本文介绍了一种新的低介电溶剂 1,2-二甲氧基丙烷 (DMP) 作为电解质溶剂。与 DME 相比,DMP 由于其空间效应增加而降低了溶剂化能力,从而促进了阴离子-Li +相互作用。2 M LiFSI-in-DMP 电解质的这种受控溶剂化结构有助于在锂金属阳极形成阴离子驱动的稳定界面和氧化稳定性,从而与广泛采用的阴极兼容,提供 Li|LiFePO 4和 Li|LiNi0.8 Co 0.1 Mn 0.1 O 2电池具有良好的循环稳定性。这些结果表明空间控制作为常用氟化的替代策略的有用性,可以微调溶剂化能力,并且通常可以设计用于实用锂金属电池的新溶剂。
更新日期:2022-12-04
中文翻译:

利用 1,2-二甲氧基丙烷的空间效应和低介电常数制造 4.3 V 锂金属电池
1,2-二甲氧基乙烷 (DME) 因其固有的还原稳定性而被广泛用作锂金属电池的电解质溶剂;然而,其低氧化稳定性对在高压锂金属电池 (LMB) 中的应用提出了重大挑战。在这个方向上,本文介绍了一种新的低介电溶剂 1,2-二甲氧基丙烷 (DMP) 作为电解质溶剂。与 DME 相比,DMP 由于其空间效应增加而降低了溶剂化能力,从而促进了阴离子-Li +相互作用。2 M LiFSI-in-DMP 电解质的这种受控溶剂化结构有助于在锂金属阳极形成阴离子驱动的稳定界面和氧化稳定性,从而与广泛采用的阴极兼容,提供 Li|LiFePO 4和 Li|LiNi0.8 Co 0.1 Mn 0.1 O 2电池具有良好的循环稳定性。这些结果表明空间控制作为常用氟化的替代策略的有用性,可以微调溶剂化能力,并且通常可以设计用于实用锂金属电池的新溶剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号