European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-12-02 , DOI: 10.1016/j.ejmech.2022.114982 Shiting Zhao 1 , Abdelsalam S Ali 2 , Xinyu Kong 3 , Yan Zhang 3 , Xiaomin Liu 4 , Melissa A Skidmore 2 , Craig M Forsyth 5 , G Paul Savage 2 , Donghai Wu 1 , Yong Xu 1 , Craig L Francis 2
|
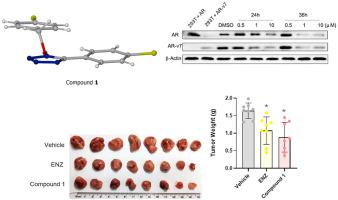
A series of 1-benzyloxy-5-phenyltetrazole derivatives and similar compounds were synthesized and evaluated for their in vitro inhibitory activity against androgen-receptor-dependent (22Rv1) and androgen-receptor independent (PC3) prostate cancer cells. The most active compounds had in vitro IC50 values against 22Rv1 cells of <50 nM and showed apparent selectivity for this cell type over PC3 cells; however, these active compounds had short half-lives when incubated with mouse liver microsomes and/or when plasma concentration was monitored during in vivo pharmacokinetic studies in mice or rats. Importantly, lead compound 1 exhibited promising inhibitory effects on cell proliferation, expression of AR and its splicing variant AR-v7 as well as AR regulated target genes in 22Rv1 cells, which are so called castration-resistant prostate cancer (CRPC) cells, and a 22Rv1 CRPC xenograft tumour model in mice. Structural changes which omitted the N–O-benzyl moiety led to dramatic or total loss of activity and S-benzylation of a cysteine derivative, as a surrogate for in vivo S-nucleophiles, by representative highly active compounds, suggested a possible chemical reactivity basis for this “activity cliff” and poor pharmacokinetic profile. However, representative highly active compounds did not inhibit a cysteine protease, indicating that the mode of activity is unlikely to be protein modification by S-benzylation. Despite our efforts to elucidate the mode of action, the mechanism remains unclear.
中文翻译:

1-苄氧基-5-苯基四唑衍生物对雄激素受体依赖性前列腺癌细胞具有高活性
合成了一系列 1-benzyloxy-5-phenyltetrazole 衍生物和类似化合物,并评估了它们对雄激素受体依赖性 (22Rv1) 和雄激素受体非依赖性 (PC3) 前列腺癌细胞的体外抑制活性。最活跃的化合物具有体外 IC 50针对 22Rv1 细胞的值 <50 nM,并且显示出对该细胞类型明显优于 PC3 细胞的选择性;然而,当与小鼠肝微粒体孵育和/或在小鼠或大鼠体内药代动力学研究期间监测血浆浓度时,这些活性化合物的半衰期较短。重要的是,先导化合物 1 对细胞增殖、AR 及其剪接变体 AR-v7 的表达以及 22Rv1 细胞(即所谓的去势抵抗性前列腺癌 (CRPC) 细胞)中 AR 调节的靶基因表现出良好的抑制作用,并且小鼠 22Rv1 CRPC 异种移植肿瘤模型。省略 N-O- 苄基部分的结构变化导致半胱氨酸衍生物的活性显着或完全丧失和 S- 苄化,作为体内 S-亲核试剂的替代物,通过代表性的高活性化合物,提出了这种“活性悬崖”和不良药代动力学特征的可能化学反应基础。然而,代表性的高活性化合物不抑制半胱氨酸蛋白酶,表明活性模式不太可能是通过 S-苄基化修饰蛋白质。尽管我们努力阐明作用方式,但机制仍不清楚。






























 京公网安备 11010802027423号
京公网安备 11010802027423号