当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Defluorinative [4 + 2] Annulation of N-Sulfonylarylamides with Ethyl 2-Diazo-3,3,3-trifluoropropanoate: Synthesis of 1,3,4-Functionalized Isoquinolines
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03501 Haosheng Li 1 , Mingjing Mei 1 , Lei Zhou 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03501 Haosheng Li 1 , Mingjing Mei 1 , Lei Zhou 1
Affiliation
|
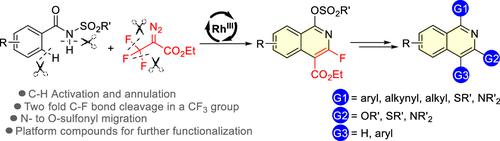
Using 2-diazo-3,3,3-trifluoropropanoate as a nontraditional two-carbon reaction partner, a Rh(III)-catalyzed defluorinative [4 + 2] annulation for the synthesis of 1,3,4-functionalized isoquinolines was developed. The reaction proceeds by sequential C–H carbenoid insertion, dual C–F bond cleavage/annulation, and N– to O–sulfonyl migration. The resultant products were converted to diverse 1,3,4-trisubstituted isoquinolines based on the functionalization of the newly installed 1-sulfonate, 2-fluoro functional handles, and/or remaining ester motif.
中文翻译:

Rh(III)-催化脱氟 [4 + 2] N-磺酰芳基酰胺与 2-重氮-3,3,3-三氟丙酸乙酯的环化:1,3,4-官能化异喹啉的合成
使用 2-diazo-3,3,3-trifluoropropanoate 作为非传统的双碳反应伙伴,开发了用于合成 1,3,4-功能化异喹啉的 Rh(III) 催化脱氟 [4 + 2] 环化。该反应通过连续的 C-H 类胡萝卜素插入、双 C-F 键断裂/环化以及 N- 到 O- 磺酰基迁移进行。基于新安装的 1-磺酸盐、2-氟官能手柄和/或剩余的酯基序的功能化,将所得产物转化为多种 1,3,4-三取代异喹啉。
更新日期:2022-12-01
中文翻译:

Rh(III)-催化脱氟 [4 + 2] N-磺酰芳基酰胺与 2-重氮-3,3,3-三氟丙酸乙酯的环化:1,3,4-官能化异喹啉的合成
使用 2-diazo-3,3,3-trifluoropropanoate 作为非传统的双碳反应伙伴,开发了用于合成 1,3,4-功能化异喹啉的 Rh(III) 催化脱氟 [4 + 2] 环化。该反应通过连续的 C-H 类胡萝卜素插入、双 C-F 键断裂/环化以及 N- 到 O- 磺酰基迁移进行。基于新安装的 1-磺酸盐、2-氟官能手柄和/或剩余的酯基序的功能化,将所得产物转化为多种 1,3,4-三取代异喹啉。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号