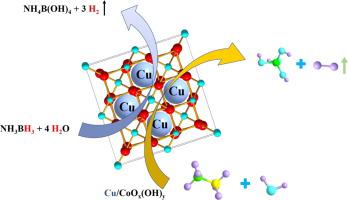
尽管已经开发了多种基于贵金属的纳米催化剂,例如 Pd、Pt、Au、Rh 和 Ru,用于从 NH 3 BH 3的水解脱氢中高效放出H 2,但非常需要设计和开发非贵金属纳米材料作为NH 3 BH 3水解脱氢生成H 2的高效纳米催化剂。在此,我们首先设计并合成了一系列 CoO x (OH) y胶体稳定的 Cu、Ni、Co 和 Fe 纳米粒子(Cu/CoO x (OH) y , Ni/CoO x (OH) y , Co/CoO x(OH) y和 Fe/CoO x (OH) y ),通过将 Cu、Ni、Co 或 Fe 纳米粒子固定在 CoO x (OH) y胶体表面,用于从 NH 3水解脱氢中高效释放 H 2 BH 3在 30 °C。可选的 Cu/CoO x (OH) y纳米杂化物在 NH 水解脱氢的H 2释放中表现出超高的催化性能,最高 TOF 为 67.5 mol(H 2 )·mol Cu -1 ·min -1 3 BH 3在 30°C。更有趣的是,Cu/CoO x (OH) y纳米杂化物催化NH 3 BH 3水解脱氢的TOF达到630.58 mol(H 2 )·mol Cu -1 ·min -1 (2789 mL(H 2 )·g cat -1 ·min -1 )在0.3 mol/L NaOH下,超过了所有或几乎所有的非贵金属纳米催化剂,甚至超过了一些贵金属纳米催化剂。比较其他常见载体,包括 Fe(OH) 3、Ni(OH) 2和 Cu(OH) 2,突出了 CoO x (OH) y的重要作用在NH 3 BH 3水解脱氢。具有 D 2 O的 2.7 的大 KIE表明水的氧-氢键断裂是 NH 3 BH 3水解脱氢中的速率决定步骤。该工作不仅为磁性纳米材料的低成本设计和合成提供了新的思路,而且为NH 3 BH 3水解脱氢制氢提供了一种高效的无贵金属纳米催化剂。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Hydrolytic dehydrogenation of NH3BH3 over Cu/CoOx(OH)y nanocomposite for H2 evolution
Although a variety of noble-metal-based nanocatalysts, such as Pd, Pt, Au, Rh, and Ru, have been developed for the efficient H2 evolution from hydrolytic dehydrogenation of NH3BH3, it is extremely desirable to design and develop noble-metal-free nanomaterials as the high-efficiency nanocatalysts for H2 generation from hydrolytic dehydrogenation of NH3BH3. Herein, we first designed and synthesized a string of CoOx(OH)y colloid-stabilized Cu, Ni, Co and Fe nanoparticles (Cu/CoOx(OH)y, Ni/CoOx(OH)y, Co/CoOx(OH)y and Fe/CoOx(OH)y), by immobilization of Cu, Ni, Co or Fe nanoparticles at the surface of CoOx(OH)y colloid, for the efficient H2 evolution from hydrolytic dehydrogenation of NH3BH3 at 30 °C. The optional Cu/CoOx(OH)y nanohybrid exhibits the super-high catalytic property, with the highest TOF of 67.5 mol(H2)·molCu-1·min−1, in the H2 evolution from hydrolytic dehydrogenation of NH3BH3 at 30 °C. More Interestingly, the TOF of Cu/CoOx(OH)y nanohybrid catalyzed hydrolytic dehydrogenation of NH3BH3 reaches to 630.58 mol(H2)·molCu-1·min−1 (2789 mL(H2)·gcat-1·min−1) under 0.3 mol/L NaOH, which exceeds all or almost all non-noble metal nanocatalysts, even some noble metal nanocatalysts. The comparison of other common supports, including Fe(OH)3, Ni(OH)2 and Cu(OH)2, has highlighted the vital role of CoOx(OH)y in NH3BH3 hydrolytic dehydrogenation. A large KIE of 2.7 with D2O has illustrated that the oxygen-hydrogen bond cleavage of water was the rate-determining step in the hydrolytic dehydrogenation of NH3BH3. This work not only provides a new explication for the cost-effective design and synthesis of magnetic nanomaterials, but also offers a high-efficiency noble-metal-free nanocatalyst for H2 evolution from hydrolytic dehydrogenation of NH3BH3.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号