当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation and Characterization of a Formally NiIV–Oxo Complex with a Triplet Ground State and Application in Oxidation Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10196 Deepika G Karmalkar 1 , Virginia A Larson 2 , Deesha D Malik 1 , Yong-Min Lee 1 , Mi Sook Seo 1 , Jin Kim 1 , Dovydas Vasiliauskas 3 , Jason Shearer 3 , Nicolai Lehnert 2 , Wonwoo Nam 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10196 Deepika G Karmalkar 1 , Virginia A Larson 2 , Deesha D Malik 1 , Yong-Min Lee 1 , Mi Sook Seo 1 , Jin Kim 1 , Dovydas Vasiliauskas 3 , Jason Shearer 3 , Nicolai Lehnert 2 , Wonwoo Nam 1
Affiliation
|
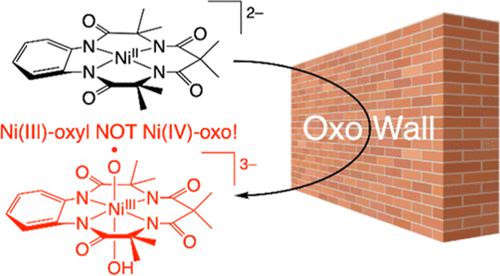
High-valent first-row transition-metal–oxo complexes are important intermediates in biologically and chemically relevant oxidative transformations of organic molecules and in the water splitting reaction in (artificial) photosynthesis. While high-valent Fe– and Mn–oxo complexes have been characterized in detail, much less is known about their analogues with late transition metals. In this study, we present the synthesis and detailed characterization of a unique mononuclear terminal Ni–O complex. This compound, [Ni(TAML)(O)(OH)]3–, is characterized by an intense charge-transfer (CT) band around 730 nm and has an St = 1 ground state, as determined by magnetic circular dichroism spectroscopy. From extended X-ray absorption fine structure (EXAFS), the Ni–O bond distance is 1.84 Å. Ni K edge XAS data indicate that the complex contains a Ni(III) center, which results from an unusually large degree of Ni–O π-bond inversion, with one hole located on the oxo ligand. The complex is therefore best described as a low-spin Ni(III) complex (S = 1/2) with a bound oxyl (O•–) ligand (S = 1/2), where the spins of Ni and oxyl are ferromagnetically coupled, giving rise to the observed St = 1 ground state. This bonding description is roughly equivalent to the presence of a Ni–O single (σ) bond. Reactivity studies show that [Ni(TAML)(O)(OH)]3– is a strong oxidant capable of oxidizing thioanisole and styrene derivatives with large negative ρ values in the Hammett plot, indicating its electrophilic nature. The intermediate also shows high reactivity in C–H bond activation of hydrocarbons with a kinetic isotope effect of 7.0(3) in xanthene oxidation.
中文翻译:

具有三重基态的形式化 NiIV-Oxo 络合物的制备和表征及其在氧化反应中的应用
高价第一行过渡金属-氧代络合物是有机分子在生物学和化学上相关的氧化转化以及(人工)光合作用中的水分解反应中的重要中间体。虽然高价 Fe- 和 Mn-oxo 络合物已被详细表征,但对它们与后过渡金属的类似物知之甚少。在这项研究中,我们介绍了一种独特的单核末端 Ni-O 络合物的合成和详细表征。这种化合物 [Ni(TAML)(O)(OH)] 3–的特征在于 730 nm 附近的强电荷转移 (CT) 带,并且具有S t= 1 基态,由磁性圆二色光谱确定。根据扩展 X 射线吸收精细结构 (EXAFS),Ni-O 键距为 1.84 Å。Ni K 边缘 XAS 数据表明该络合物包含一个 Ni(III) 中心,这是由异常大程度的 Ni-O π 键反转产生的,其中一个孔位于氧代配体上。因此,该络合物最好被描述为低自旋 Ni(III) 络合物 ( S = 1/2) 与结合的氧基 (O •– ) 配体 ( S = 1/2),其中 Ni 和氧基的自旋是铁磁性的耦合,产生观察到的S t = 1 基态。这种成键描述大致等同于 Ni–O 单 (σ) 键的存在。反应性研究表明 [Ni(TAML)(O)(OH)]3–是一种强氧化剂,能够氧化硫代苯甲醚和苯乙烯衍生物,在哈米特图中具有较大的负 ρ 值,表明其亲电子性质。该中间体还在碳氢化合物的 C-H 键活化中表现出高反应性,在呫吨氧化中具有 7.0(3) 的动力学同位素效应。
更新日期:2022-12-01
中文翻译:

具有三重基态的形式化 NiIV-Oxo 络合物的制备和表征及其在氧化反应中的应用
高价第一行过渡金属-氧代络合物是有机分子在生物学和化学上相关的氧化转化以及(人工)光合作用中的水分解反应中的重要中间体。虽然高价 Fe- 和 Mn-oxo 络合物已被详细表征,但对它们与后过渡金属的类似物知之甚少。在这项研究中,我们介绍了一种独特的单核末端 Ni-O 络合物的合成和详细表征。这种化合物 [Ni(TAML)(O)(OH)] 3–的特征在于 730 nm 附近的强电荷转移 (CT) 带,并且具有S t= 1 基态,由磁性圆二色光谱确定。根据扩展 X 射线吸收精细结构 (EXAFS),Ni-O 键距为 1.84 Å。Ni K 边缘 XAS 数据表明该络合物包含一个 Ni(III) 中心,这是由异常大程度的 Ni-O π 键反转产生的,其中一个孔位于氧代配体上。因此,该络合物最好被描述为低自旋 Ni(III) 络合物 ( S = 1/2) 与结合的氧基 (O •– ) 配体 ( S = 1/2),其中 Ni 和氧基的自旋是铁磁性的耦合,产生观察到的S t = 1 基态。这种成键描述大致等同于 Ni–O 单 (σ) 键的存在。反应性研究表明 [Ni(TAML)(O)(OH)]3–是一种强氧化剂,能够氧化硫代苯甲醚和苯乙烯衍生物,在哈米特图中具有较大的负 ρ 值,表明其亲电子性质。该中间体还在碳氢化合物的 C-H 键活化中表现出高反应性,在呫吨氧化中具有 7.0(3) 的动力学同位素效应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号