当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pairing of Aqueous and Nonaqueous Electrosynthetic Reactions Enabled by a Redox Reservoir Electrode
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09632
Katelyn H Michael 1 , Zhi-Ming Su 1 , Rui Wang 1 , Hongyuan Sheng 1 , Wenjie Li 1 , Fengmei Wang 1, 2 , Shannon S Stahl 1 , Song Jin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09632
Katelyn H Michael 1 , Zhi-Ming Su 1 , Rui Wang 1 , Hongyuan Sheng 1 , Wenjie Li 1 , Fengmei Wang 1, 2 , Shannon S Stahl 1 , Song Jin 1
Affiliation
|
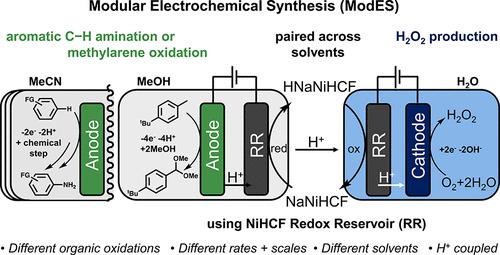
Paired electrolysis methods are appealing for chemical synthesis because they generate valuable products at both electrodes; however, development of such reactions is complicated by the need for both half-reactions to proceed under mutually compatible conditions. Here, a modular electrochemical synthesis (ModES) strategy bypasses these constraints using a “redox reservoir” (RR) to pair electrochemical half-reactions across aqueous and nonaqueous solvents. Electrochemical oxidation reactions in organic solvents, the conversion of 4-t-butyltoluene to benzylic dimethyl acetal and aldehyde in methanol or the oxidative C–H amination of naphthalene in acetonitrile, and the reduction of oxygen to hydrogen peroxide in water were paired using nickel hexacyanoferrate as an RR that can selectively store and release protons (and electrons) while serving as the counter electrode for these reactions. Selective proton transport through the RR is optimized and confirmed to enable the ion balance, and thus the successful pairing, between redox half-reactions that proceed with different rates, on different scales, and in different solvents (methanol, acetonitrile, and water).
中文翻译:

氧化还原储电极实现水性和非水性电合成反应的配对
配对电解方法对于化学合成很有吸引力,因为它们在两个电极上都能产生有价值的产物。然而,由于两个半反应需要在相互兼容的条件下进行,因此此类反应的发展变得复杂。在这里,模块化电化学合成(ModES)策略使用“氧化还原库”(RR)来配对水性和非水性溶剂中的电化学半反应,从而绕过了这些限制。使用六氰基铁酸镍将有机溶剂中的电化学氧化反应、4-叔丁基甲苯在甲醇中转化为苄基二甲缩醛和醛或萘在乙腈中的氧化C-H胺化反应以及水中氧还原成过氧化氢反应进行配对作为 RR,可以选择性地存储和释放质子(和电子),同时充当这些反应的对电极。通过 RR 的选择性质子传输经过优化和确认,可实现离子平衡,从而实现以不同速率、不同规模、在不同溶剂(甲醇、乙腈和水)中进行的氧化还原半反应之间的成功配对。
更新日期:2022-11-30
中文翻译:

氧化还原储电极实现水性和非水性电合成反应的配对
配对电解方法对于化学合成很有吸引力,因为它们在两个电极上都能产生有价值的产物。然而,由于两个半反应需要在相互兼容的条件下进行,因此此类反应的发展变得复杂。在这里,模块化电化学合成(ModES)策略使用“氧化还原库”(RR)来配对水性和非水性溶剂中的电化学半反应,从而绕过了这些限制。使用六氰基铁酸镍将有机溶剂中的电化学氧化反应、4-叔丁基甲苯在甲醇中转化为苄基二甲缩醛和醛或萘在乙腈中的氧化C-H胺化反应以及水中氧还原成过氧化氢反应进行配对作为 RR,可以选择性地存储和释放质子(和电子),同时充当这些反应的对电极。通过 RR 的选择性质子传输经过优化和确认,可实现离子平衡,从而实现以不同速率、不同规模、在不同溶剂(甲醇、乙腈和水)中进行的氧化还原半反应之间的成功配对。

































 京公网安备 11010802027423号
京公网安备 11010802027423号