The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2022-11-30 , DOI: 10.1016/j.jct.2022.106972 Qirui Guo , Weizhong Shi , Hongkun Zhao , Wanxin Li , Ali Farajtabar
|
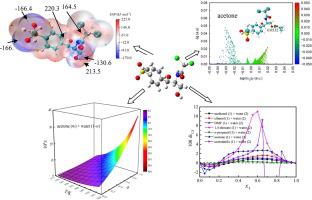
This study investigated the thermodynamic equilibrium solubility and its model correlation of thiamphenicol in aqueous solutions of n-propanol/acetone/acetonitrile as well as the solvation behavior of the compound. To explain the electrostatic properties of the basicity and the acidity of thiamphenicol molecule, a quantitative study of the molecular surface and a Hirshfeld surface analysis were utilized. These studies revealed that oxygen atoms in > SO2 and > C O groups are the most important electrophilic sites, and hydrogen atoms in –OH groups are the most important nucleophilic sites. An independent gradient model that was on the basis of the Hirshfeld partition was used to qualitatively clarify the inter-molecular interactions occurred between thiamphenicol and various solvents. At 101.1 kPa and temperatures ranging from 278.15 to 318.15 K with increments of 5 K, the isothermal dissolution technique was utilized to investigate the solubilities of this drug. The Apelblat equation, the modified van't Hoff Jouyban–Acree model, the Jouyban–Acree model, and the modified Wilson model all gave good findings for the solubility connection, with a relative average deviance of 3.41 percent. The extended Hildebrand solubility approach was used to inspect the solvation behavior at 298.15 K. The inverse Kirkwood-Buff integrals method was utilized in order to carry out the task of quantifying the thiamphenicol solvation that was favored. Positive solvation values in acetone/acetonitrile-rich and -middle solution compositions show that acetone/acetonitrile preferentially solvates thiamphenicol.
O groups are the most important electrophilic sites, and hydrogen atoms in –OH groups are the most important nucleophilic sites. An independent gradient model that was on the basis of the Hirshfeld partition was used to qualitatively clarify the inter-molecular interactions occurred between thiamphenicol and various solvents. At 101.1 kPa and temperatures ranging from 278.15 to 318.15 K with increments of 5 K, the isothermal dissolution technique was utilized to investigate the solubilities of this drug. The Apelblat equation, the modified van't Hoff Jouyban–Acree model, the Jouyban–Acree model, and the modified Wilson model all gave good findings for the solubility connection, with a relative average deviance of 3.41 percent. The extended Hildebrand solubility approach was used to inspect the solvation behavior at 298.15 K. The inverse Kirkwood-Buff integrals method was utilized in order to carry out the task of quantifying the thiamphenicol solvation that was favored. Positive solvation values in acetone/acetonitrile-rich and -middle solution compositions show that acetone/acetonitrile preferentially solvates thiamphenicol.
中文翻译:

甲砜霉素在正丙醇/丙酮/乙腈水性共溶剂中的平衡溶解度、非共价相互作用和溶剂化热力学
本研究调查了甲砜霉素在正丙醇/丙酮/乙腈水溶液中的热力学平衡溶解度及其模型相关性,以及该化合物的溶剂化行为。为了解释甲砜霉素分子的碱性和酸性的静电特性,利用了分子表面的定量研究和 Hirshfeld 表面分析。这些研究表明 >SO 2和 >C中的氧原子 O基团是最重要的亲电位点,-OH基团中的氢原子是最重要的亲核位点。基于 Hirshfeld 分区的独立梯度模型用于定性阐明甲砜霉素与各种溶剂之间发生的分子间相互作用。在 101.1 kPa 和 278.15 至 318.15 K 的温度范围内,增量为 5 K,等温溶解技术用于研究该药物的溶解度。Apelblat 方程、修正的 van't Hoff Jouyban–Acree 模型、Jouyban–Acree 模型和修正的 Wilson 模型都给出了溶解度关联的良好结果,相对平均偏差为 3.41%。扩展的希尔德布兰德溶解度方法用于检查 298.15 K 下的溶剂化行为。使用逆 Kirkwood-Buff 积分方法来执行量化受欢迎的甲砜霉素溶剂化的任务。富含丙酮/乙腈和中间溶液组合物中的正溶剂化值表明丙酮/乙腈优先溶剂化甲砜霉素。
O基团是最重要的亲电位点,-OH基团中的氢原子是最重要的亲核位点。基于 Hirshfeld 分区的独立梯度模型用于定性阐明甲砜霉素与各种溶剂之间发生的分子间相互作用。在 101.1 kPa 和 278.15 至 318.15 K 的温度范围内,增量为 5 K,等温溶解技术用于研究该药物的溶解度。Apelblat 方程、修正的 van't Hoff Jouyban–Acree 模型、Jouyban–Acree 模型和修正的 Wilson 模型都给出了溶解度关联的良好结果,相对平均偏差为 3.41%。扩展的希尔德布兰德溶解度方法用于检查 298.15 K 下的溶剂化行为。使用逆 Kirkwood-Buff 积分方法来执行量化受欢迎的甲砜霉素溶剂化的任务。富含丙酮/乙腈和中间溶液组合物中的正溶剂化值表明丙酮/乙腈优先溶剂化甲砜霉素。





























 京公网安备 11010802027423号
京公网安备 11010802027423号