Toxicology Letters ( IF 2.9 ) Pub Date : 2022-11-29 , DOI: 10.1016/j.toxlet.2022.11.019 Shuyi Gu 1 , Gaosong Wu 1 , Dong Lu 1 , Yu Wang 2 , Liming Tang 2 , Weidong Zhang 1
|
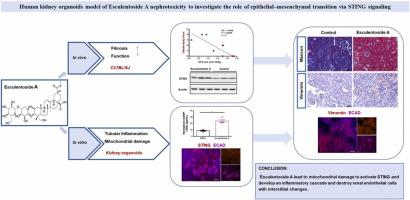
Radix Phytolaccae (RP) has a long medicinal history and is commonly used to treat systemic edema and ascites in Asia. Although RP is known to cause nephrotoxicity, the role of its main constituent, Esculentoside A (EsA), in nephrotoxicity remains undetermined. We used kidney organoids derived from human inducible pluripotent stem cells (iPSCs) to model EsA nephrotoxicity accurately. Kidney organoids were differentiated and treated with EsA at doses of 0, 15, 30, or 60 μM for 48 h. The in vitro model was compared to a mouse model of EsA nephrotoxicity (intraperitoneally injected, 25 mg·kg−1). The mechanisms were investigated. Cell viability decreased dose-dependently after treatment with EsA. As polarity was lost, tubular cells decreased, similar to mouse EsA nephrotoxicity with upregulated vimentin expression and a stimulator of the interferon gene (STING). Furthermore, 60 μM EsA could induce endothelial inflammation, lead to mitochondrial damage and activate STING by translocating mtDNA into the cytoplasm to develop an inflammatory cascade and destroy renal endothelial cells with interstitial changes. The data suggest that kidney organoids derived from iPSCs are promising for investigating nephrotoxicity. EsA nephrotoxicity involves the epithelial-mesenchymal transition via STING signaling.
中文翻译:

Esculentoside A 肾毒性人肾类器官模型通过 STING 信号研究上皮-间质转化的作用
商陆 (RP) 具有悠久的药用历史,在亚洲常用于治疗全身性水肿和腹水。尽管已知 RP 会引起肾毒性,但其主要成分七叶草苷 A (EsA) 在肾毒性中的作用仍未确定。我们使用源自人类诱导性多能干细胞 (iPSC) 的肾脏类器官来准确模拟 EsA 肾毒性。肾脏类器官被分化并用 EsA 以 0、15、30 或 60 μM 的剂量处理 48 小时。将体外模型与 EsA 肾毒性小鼠模型(腹膜内注射,25 mg·kg −1). 研究了这些机制。在用 EsA 处理后,细胞活力呈剂量依赖性降低。随着极性丢失,肾小管细胞减少,类似于小鼠 EsA 肾毒性,波形蛋白表达上调和干扰素基因刺激物 (STING)。此外,60 μM EsA 可诱导内皮炎症,导致线粒体损伤并通过将 mtDNA 转移到细胞质中激活 STING,从而形成炎症级联反应并破坏具有间质变化的肾内皮细胞。数据表明,源自 iPSC 的肾脏类器官有望用于研究肾毒性。EsA 肾毒性涉及通过 STING 信号传导的上皮-间质转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号