Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pairing d-Band Center of Metal Sites with π-Orbital of Alkynes for Efficient Electrocatalytic Alkyne Semi-Hydrogenation
Small ( IF 13.0 ) Pub Date : 2022-11-29 , DOI: 10.1002/smll.202205845 Jinjin Li 1 , Ying Guo 1 , Siyu Chang 1 , Jin Lin 1 , You Wang 1 , Zhenpeng Liu 2 , Yafei Wu 1 , Jian Zhang 1, 2
Small ( IF 13.0 ) Pub Date : 2022-11-29 , DOI: 10.1002/smll.202205845 Jinjin Li 1 , Ying Guo 1 , Siyu Chang 1 , Jin Lin 1 , You Wang 1 , Zhenpeng Liu 2 , Yafei Wu 1 , Jian Zhang 1, 2
Affiliation
|
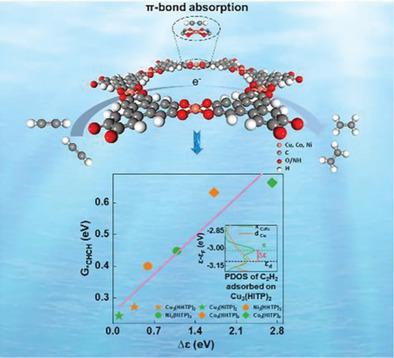
Electrocatalytic alkyne semi-hydrogenation has attracted ever-growing attention as a promising alternative to traditional thermocatalytic hydrogenation. However, the correlation between the structure of active sites and electrocatalytic performance still remains elusive. Herein, the energy difference (∆ε) between the d-band center of metal sites and π orbital of alkynes as a key descriptor for correlating the intrinsic electrocatalytic activity is reported. With two-dimensional conductive metal organic frameworks as the model electrocatalysts, theoretical and experimental investigations reveal that the decreased ∆ε induces the strengthened d–π orbitals interaction, which thus enhances acetylene π-adsorption and accelerates subsequent hydrogenation kinetics. As a result, Cu3(HITP)2 featuring the smallest ∆ε (0.10 eV) delivers the highest turnover frequency of 0.36 s−1, which is about 124 times higher than 2.9 × 10−3 s−1 for Co3(HITP)2 with the largest ∆ε of 2.71 eV. Meanwhile, Cu3(HITP)2 presents a high ethylene partial current density of −124 mA cm−2 and a large ethylene Faradaic efficiency of 99.3% at −0.9 V versus RHE. This work will spark the rapid exploration of high-activity alkyne semi-hydrogenation catalysts.
中文翻译:

将金属位点的 d 带中心与炔烃的 π 轨道配对以实现高效电催化炔烃半氢化
电催化炔烃半加氢作为传统热催化加氢的有前途的替代品,引起了越来越多的关注。然而,活性位点结构与电催化性能之间的相关性仍然不清楚。在此,报告了金属位点的 d 带中心与炔烃的 π 轨道之间的能量差 (Δε) 作为关联本征电催化活性的关键描述符。以二维导电金属有机骨架作为模型电催化剂,理论和实验研究表明,降低的 Δε 会导致增强的 d-π 轨道相互作用,从而增强乙炔的 π-吸附并加速后续的氢化动力学。结果,Cu 3 (HITP) 2具有最小 Δε (0.10 eV) 的最高转换频率为 0.36 s −1 ,比Co 3 (HITP) 2的 2.9 × 10 −3 s −1高约 124 倍,最大 Δε 为 2.71 eV . 同时,与 RHE 相比,Cu 3 (HITP) 2呈现 -124 mA cm -2的高乙烯分电流密度和 -0.9 V 下 99.3% 的大乙烯法拉第效率。这项工作将激发对高活性炔烃半加氢催化剂的快速探索。
更新日期:2022-11-29
中文翻译:

将金属位点的 d 带中心与炔烃的 π 轨道配对以实现高效电催化炔烃半氢化
电催化炔烃半加氢作为传统热催化加氢的有前途的替代品,引起了越来越多的关注。然而,活性位点结构与电催化性能之间的相关性仍然不清楚。在此,报告了金属位点的 d 带中心与炔烃的 π 轨道之间的能量差 (Δε) 作为关联本征电催化活性的关键描述符。以二维导电金属有机骨架作为模型电催化剂,理论和实验研究表明,降低的 Δε 会导致增强的 d-π 轨道相互作用,从而增强乙炔的 π-吸附并加速后续的氢化动力学。结果,Cu 3 (HITP) 2具有最小 Δε (0.10 eV) 的最高转换频率为 0.36 s −1 ,比Co 3 (HITP) 2的 2.9 × 10 −3 s −1高约 124 倍,最大 Δε 为 2.71 eV . 同时,与 RHE 相比,Cu 3 (HITP) 2呈现 -124 mA cm -2的高乙烯分电流密度和 -0.9 V 下 99.3% 的大乙烯法拉第效率。这项工作将激发对高活性炔烃半加氢催化剂的快速探索。































 京公网安备 11010802027423号
京公网安备 11010802027423号