Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-11-28 , DOI: 10.1016/j.jhazmat.2022.130502 Kang Liu 1 , Mengmeng Wang 1 , Qiaozhi Zhang 2 , Zibo Xu 2 , Claudia Labianca 2 , Michael Komárek 3 , Bin Gao 4 , Daniel C W Tsang 1
|
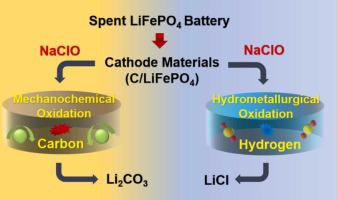
Oxidative extraction has become an economically viable option for recycling lithium (Li) from spent lithium iron phosphate (LiFePO4) batteries. In this study, the releases behaviour of Li from spent LiFePO4 batteries under different oxidizing conditions was investigated with sodium hypochlorite (NaClO) as the solid oxidant. We revealed that, due to the intervention of graphitic carbon, the generated species of Li in mechanochemical oxidation (NaClO:LiFePO4 at a molar ratio of 2:1, 5 min, and 600 rpm) was lithium carbonate (Li2CO3). The graphite layer provided a channel for the conversion of Li species released by mechanochemical oxidation. While in hydrometallurgical oxidation (NaClO:LiFePO4 at a molar ratio of 2:1 and 12.5 min), the presence of hydrogen species led to the formation of lithium chloride (LiCl). Moreover, life cycle assessment (LCA) demonstrated that for recycling 1.0 kg of spent LiFePO4 batteries, mechanochemical and hydrometallurgical oxidation could reduce carbon footprints by 2.81 kg CO2 eq and 2.88 kg CO2 eq, respectively. Our results indicate that the oxidative environment determines the release pathway of Li from the spent LiFePO4 cathode material, thereby regulating the product forms of Li and environmental impacts. This study can provide key technical guidance for Li recycling from spent LiFePO4 batteries.
中文翻译:

废磷酸铁锂正极材料在不同氧化环境下的回收机制展望
氧化萃取已成为从废磷酸铁锂 (LiFePO 4 ) 电池中回收锂 (Li) 的一种经济可行的选择。本研究以次氯酸钠(NaClO)为固体氧化剂,研究了LiFePO 4废电池在不同氧化条件下的释放行为。我们发现,由于石墨碳的介入,Li在机械化学氧化(NaClO:LiFePO 4摩尔比为2:1、5分钟和600 rpm)中生成的物质是碳酸锂(Li 2 CO 3) . 石墨层为机械化学氧化释放的锂物种的转化提供了通道。而在湿法冶金氧化(NaClO:LiFePO 4在 2:1 和 12.5 分钟的摩尔比下,氢物种的存在导致氯化锂 (LiCl) 的形成。此外,生命周期评估 (LCA) 表明,对于回收 1.0 kg 废 LiFePO 4电池,机械化学和湿法冶金氧化可分别减少 2.81 kg CO 2 eq 和 2.88 kg CO 2 eq 的碳足迹。我们的结果表明,氧化环境决定了 Li 从废 LiFePO 4正极材料中的释放途径,从而调节了 Li 的产物形式和环境影响。该研究可为废旧LiFePO 4电池的锂回收提供关键技术指导。















































 京公网安备 11010802027423号
京公网安备 11010802027423号