当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Branched-Selective Hydroacylation of Alkenes via Photoredox Cobalt and N-Heterocyclic Carbene Cooperative Triple Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-29 , DOI: 10.1021/acscatal.2c04970 Xiangzhang Tao 1 , Qing Wang 1 , Lingyu Kong 1 , Shengyang Ni 1 , Yi Pan 1 , Yi Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-29 , DOI: 10.1021/acscatal.2c04970 Xiangzhang Tao 1 , Qing Wang 1 , Lingyu Kong 1 , Shengyang Ni 1 , Yi Pan 1 , Yi Wang 1
Affiliation
|
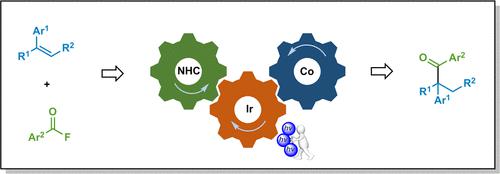
A Markovnikov-selective hydroacylation of alkenes has been achieved via the synergistic merger cobalt, photoredox and N-heterocyclic carbene catalysis. The closely incorporated catalytic cycles allow for Co(III) generation by photochemical oxidation instead of chemical oxidants or anodizing process. This mild, operationally simple protocol converts a wide variety of commercially available alkenes and aroyl fluorides into the corresponding ketones in high yield with branched selectivity.
中文翻译:

光氧化还原钴和 N-杂环卡宾协同三重催化对烯烃进行支链选择性加氢酰化
通过钴、光氧化还原和N-杂环卡宾催化的协同合并,实现了烯烃的马尔可夫尼科夫选择性加氢酰化。紧密结合的催化循环允许通过光化学氧化而不是化学氧化剂或阳极氧化过程生成 Co(III)。这种温和、操作简单的方案可将各种市售烯烃和芳酰氟以高产率和分支选择性转化为相应的酮。
更新日期:2022-11-29
中文翻译:

光氧化还原钴和 N-杂环卡宾协同三重催化对烯烃进行支链选择性加氢酰化
通过钴、光氧化还原和N-杂环卡宾催化的协同合并,实现了烯烃的马尔可夫尼科夫选择性加氢酰化。紧密结合的催化循环允许通过光化学氧化而不是化学氧化剂或阳极氧化过程生成 Co(III)。这种温和、操作简单的方案可将各种市售烯烃和芳酰氟以高产率和分支选择性转化为相应的酮。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号