当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Solvent Effect Analysis of N-Trityl Olmesartan Ethyl Ester in 13 Organic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.jced.2c00366 Peixian Li 1 , Li Chen 1 , Cunbin Du 1 , Rongrong Li 1 , Yang Yu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.jced.2c00366 Peixian Li 1 , Li Chen 1 , Cunbin Du 1 , Rongrong Li 1 , Yang Yu 1
Affiliation
|
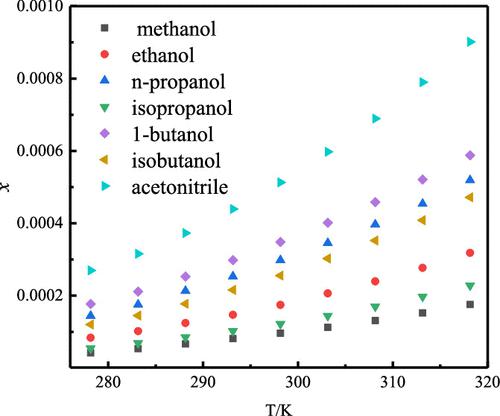
N-Trityl olmesartan ethyl ester is a key intermediate of the launched angiotensin II receptor blocker olmesartan medoxomil. The aim of this work was to measure the solubility data of N-trityl olmesartan ethyl ester in different organic solvents at various temperatures. Experimental mole fraction solubility values of N-trityl olmesartan ethyl ester were recorded, with the largest observed for cyclohexanone (2.110 × 10–2), followed by N,N-dimethylacetamide (DMAC, 1.476 × 10–2), toluene (1.237 × 10–2), ethyl acetate (6.993 × 10–3), N,N-dimethylformamide (DMF, 5.527 × 10–3), acetone (4.174 × 10–3), acetonitrile (9.013 × 10–4), 1-butanol (5.878 × 10–4), n-propanol (5.192 × 10–4), isobutanol (4.717 × 10–4), ethanol (3.181 × 10–4), isopropanol (2.281 × 10–4), and methanol (1.755 × 10–4) at 318.15 K. A similar sequence of solubility data was also observed at other temperatures. The KAT-LSER model was applied to investigate the solvent effect on the basis of linear solvation energy relationships. The correlation results indicated that the modified Apelblat equation was more suitable to evaluate the dissolution behavior of N-trityl olmesartan ethyl ester in this work. Based on these results, cyclohexanone and DMAC were optimized as suitable organic solvents in the purification and recrystallization of N-trityl olmesartan ethyl ester in pharmaceutical industries.
中文翻译:

N-三苯甲基奥美沙坦乙酯在13种有机溶剂中的溶解度测定及溶剂效应分析
N -三苯甲基奥美沙坦乙酯是已上市的血管紧张素 II 受体阻滞剂奥美沙坦酯的关键中间体。这项工作的目的是测量N-三苯甲基奥美沙坦乙酯在不同温度下在不同有机溶剂中的溶解度数据。记录了N-三苯甲基奥美沙坦乙酯的实验摩尔分数溶解度值,观察到的最大的是环己酮 (2.110 × 10 –2 ),其次是N , N-二甲基乙酰胺 (DMAC, 1.476 × 10 –2 )、甲苯 (1.237 × 10 –2 ), 乙酸乙酯 (6.993 × 10 –3 ), N , N-二甲基甲酰胺 (DMF, 5.527 × 10–3 ), 丙酮 (4.174 × 10 –3 ), 乙腈 (9.013 × 10 –4 ), 1-丁醇 (5.878 × 10 –4 ) ,正丙醇 (5.192 × 10 –4 ), 异丁醇 (4.717 × 10 –4 ) 4 )、乙醇 (3.181 × 10 –4 )、异丙醇 (2.281 × 10 –4 ) 和甲醇 (1.755 × 10 –4 ) 在 318.15 K 下。在其他温度下也观察到类似的溶解度数据序列。基于线性溶剂化能关系,应用 KAT-LSER 模型研究溶剂效应。相关性结果表明修正的 Apelblat 方程更适合评价N的溶出行为-这项工作中的三苯甲基奥美沙坦乙酯。基于这些结果,优化了环己酮和DMAC作为制药工业中N-三苯甲基奥美沙坦乙酯纯化和重结晶的合适有机溶剂。
更新日期:2022-11-28
中文翻译:

N-三苯甲基奥美沙坦乙酯在13种有机溶剂中的溶解度测定及溶剂效应分析
N -三苯甲基奥美沙坦乙酯是已上市的血管紧张素 II 受体阻滞剂奥美沙坦酯的关键中间体。这项工作的目的是测量N-三苯甲基奥美沙坦乙酯在不同温度下在不同有机溶剂中的溶解度数据。记录了N-三苯甲基奥美沙坦乙酯的实验摩尔分数溶解度值,观察到的最大的是环己酮 (2.110 × 10 –2 ),其次是N , N-二甲基乙酰胺 (DMAC, 1.476 × 10 –2 )、甲苯 (1.237 × 10 –2 ), 乙酸乙酯 (6.993 × 10 –3 ), N , N-二甲基甲酰胺 (DMF, 5.527 × 10–3 ), 丙酮 (4.174 × 10 –3 ), 乙腈 (9.013 × 10 –4 ), 1-丁醇 (5.878 × 10 –4 ) ,正丙醇 (5.192 × 10 –4 ), 异丁醇 (4.717 × 10 –4 ) 4 )、乙醇 (3.181 × 10 –4 )、异丙醇 (2.281 × 10 –4 ) 和甲醇 (1.755 × 10 –4 ) 在 318.15 K 下。在其他温度下也观察到类似的溶解度数据序列。基于线性溶剂化能关系,应用 KAT-LSER 模型研究溶剂效应。相关性结果表明修正的 Apelblat 方程更适合评价N的溶出行为-这项工作中的三苯甲基奥美沙坦乙酯。基于这些结果,优化了环己酮和DMAC作为制药工业中N-三苯甲基奥美沙坦乙酯纯化和重结晶的合适有机溶剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号