Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Euscapholide and Tetraketide via Prins Cyclisation and Ring-Closing Metathesis
SynOpen ( IF 2.0 ) Pub Date : 2022-11-24 , DOI: 10.1055/s-0042-1751381 Dhanraj O. Biradar 1, 2 , Basi V. Subba Reddy 1 , Yogesh D. Mane 3
中文翻译:

普林斯环化和闭环复分解立体选择性合成芥子内酯和四酮化合物
更新日期:2022-11-25
SynOpen ( IF 2.0 ) Pub Date : 2022-11-24 , DOI: 10.1055/s-0042-1751381 Dhanraj O. Biradar 1, 2 , Basi V. Subba Reddy 1 , Yogesh D. Mane 3
Affiliation
|
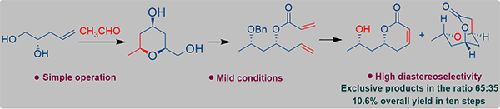
A concise and diastereoselective total synthesis of tetraketide and euscapholide is described in ten steps in 10.6% overall yield from acetaldehyde and (S)-pent-4-ene-1,2-diol. Jacobsen hydrolytic kinetic resolution, Prins cyclization, ring-closing metathesis and oxa-Michael addition reactions are the key steps involved in the synthesis.
中文翻译:

普林斯环化和闭环复分解立体选择性合成芥子内酯和四酮化合物
以乙醛和 ( S )-pent-4-ene-1,2-diol为原料,分十步以 10.6% 的总收率描述了四酮内酯和 euscapholide 的简明非对映选择性全合成。Jacobsen 水解动力学拆分、Prins 环化、闭环复分解和 oxa-Michael 加成反应是合成中涉及的关键步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号