Cell Chemical Biology ( IF 6.6 ) Pub Date : 2022-11-23 , DOI: 10.1016/j.chembiol.2022.11.003
Hengrui Liu 1 , Farhad Forouhar 2 , Annie J Lin 3 , Qian Wang 3 , Vasiliki Polychronidou 3 , Rajesh Kumar Soni 4 , Xin Xia 3 , Brent R Stockwell 5
|
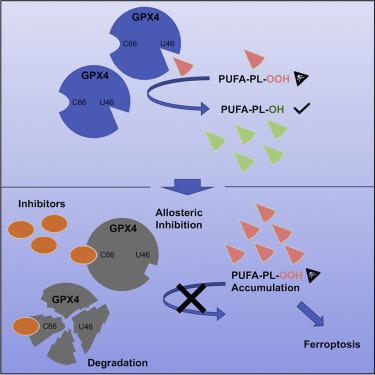
Encouraged by the dependence of drug-resistant, metastatic cancers on GPX4, we examined biophysical mechanisms of GPX4 inhibition, which revealed an unexpected allosteric site. We found that this site was involved in native regeneration of GPX4 under low glutathione conditions. Covalent binding of inhibitors to this allosteric site caused a conformational change, inhibition of activity, and subsequent cellular GPX4 protein degradation. To verify this site in an unbiased manner, we screened a library of compounds and identified and validated that an additional compound can covalently bind in this allosteric site, inhibiting and degrading GPX4. We determined co-crystal structures of six different inhibitors bound in this site. We have thus identified an allosteric mechanism for small molecules targeting aggressive cancers dependent on GPX4.
中文翻译:

GPX4 小分子变构抑制剂
受耐药性转移性癌症对 GPX4 依赖性的启发,我们研究了 GPX4 抑制的生物物理机制,结果揭示了一个意想不到的变构位点。我们发现该位点参与低谷胱甘肽条件下 GPX4 的天然再生。抑制剂与该变构位点的共价结合引起构象变化、活性抑制以及随后的细胞 GPX4 蛋白降解。为了以公正的方式验证该位点,我们筛选了化合物库,并鉴定并验证了另一种化合物可以共价结合在该变构位点上,从而抑制和降解 GPX4。我们确定了结合在该位点的六种不同抑制剂的共晶结构。因此,我们确定了一种依赖 GPX4 的小分子靶向侵袭性癌症的变构机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号