当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Organocatalytic Asymmetric Synthesis of Indolyl-Pyrroloindoles Bearing Both Axial and Central Chirality
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-23 , DOI: 10.1021/acs.joc.2c02303 Hai-Qing Wang 1 , Shu-Fang Wu 1 , Jun-Ru Yang 1 , Yu-Chen Zhang 1 , Feng Shi 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-23 , DOI: 10.1021/acs.joc.2c02303 Hai-Qing Wang 1 , Shu-Fang Wu 1 , Jun-Ru Yang 1 , Yu-Chen Zhang 1 , Feng Shi 1, 2
Affiliation
|
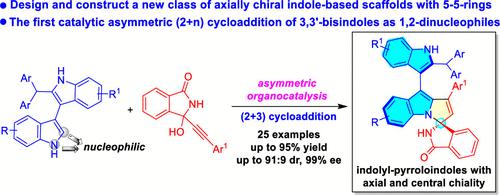
An axially chiral indolyl-pyrroloindole scaffold, a new member of axially chiral indole-based scaffolds, has been designed, and the catalytic asymmetric construction of this scaffold has been established by the strategy of organocatalytic asymmetric (2 + 3) cycloaddition of 3,3′-bisindoles with isoindolinone-based propargylic alcohols. By this approach, a series of indolyl-pyrroloindole derivatives bearing both axial chirality and central chirality were synthesized in high yields with excellent diastereo- and enantioselectivities (up to 95% yield, 91:9 dr, 99% ee). This reaction not only realizes the first catalytic asymmetric (2 + n) cycloaddition of 3,3′-bisindoles as 1,2-dinucleophiles but also provides a new strategy for atroposelective construction of axially chiral indole-based scaffolds bearing five–five-membered rings, thus solving the challenges in constructing this class of axially chiral indole-based scaffolds.
中文翻译:

具有轴向手性和中心手性的吲哚基吡咯并吲哚的设计与有机催化不对称合成
设计了轴向手性吲哚基吡咯并吲哚支架,这是轴向手性吲哚基支架的新成员,并通过3,3的有机催化不对称(2+3)环加成策略建立了该支架的催化不对称结构。 '-双吲哚与基于异吲哚酮的炔丙醇。通过这种方法,一系列具有轴向手性和中心手性的吲哚基吡咯并吲哚衍生物以高产率合成,具有优异的非对映和对映选择性(产率高达95%,91:9 dr,99% ee)。该反应不仅实现了第一个催化不对称(2 + n)3,3'-双吲哚作为1,2-二亲核试剂的环加成反应,同时也为具有五元环的轴向手性吲哚基支架的逆向选择性构建提供了新的策略,从而解决了构建此类轴向手性支架的挑战基于吲哚的支架。
更新日期:2022-11-23
中文翻译:

具有轴向手性和中心手性的吲哚基吡咯并吲哚的设计与有机催化不对称合成
设计了轴向手性吲哚基吡咯并吲哚支架,这是轴向手性吲哚基支架的新成员,并通过3,3的有机催化不对称(2+3)环加成策略建立了该支架的催化不对称结构。 '-双吲哚与基于异吲哚酮的炔丙醇。通过这种方法,一系列具有轴向手性和中心手性的吲哚基吡咯并吲哚衍生物以高产率合成,具有优异的非对映和对映选择性(产率高达95%,91:9 dr,99% ee)。该反应不仅实现了第一个催化不对称(2 + n)3,3'-双吲哚作为1,2-二亲核试剂的环加成反应,同时也为具有五元环的轴向手性吲哚基支架的逆向选择性构建提供了新的策略,从而解决了构建此类轴向手性支架的挑战基于吲哚的支架。































 京公网安备 11010802027423号
京公网安备 11010802027423号