Chem ( IF 19.1 ) Pub Date : 2022-11-22 , DOI: 10.1016/j.chempr.2022.11.001 Kangjiang Liang , Xipan Li , Delian Wei , Cuihua Jin , Chuanwang Liu , Chengfeng Xia
|
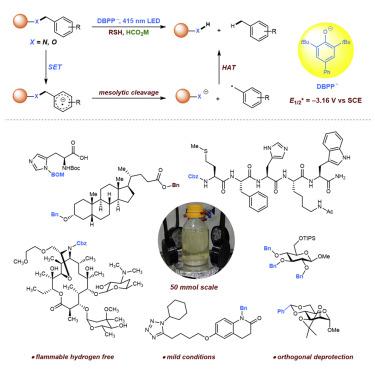
The benzyl group and its derivatives are the most frequently and widely utilized protective groups in organic synthesis and are typically removed by traditional transition-metal catalyzed hydrogenolysis or Birch reduction. The hydrogenolysis is limited by compatibility for functional groups and by the flammability and explosibility of hydrogen, while the Birch reduction suffers from flammable alkalis and harsh conditions. Here, we report that the benzyl-derived protecting groups are easily removed via visible light irradiation under mild conditions. We found that an excited phenolate-type photocatalyst efficiently transfers an electron to the benzene ring of benzyl-derived protecting groups to generate a phenyl radical anion. The benzyl C–N or C–O bond is then mesolytically cleaved by releasing a benzyl radical. This method enables efficient deprotection of the benzyl group on amides, arylamines, ether, ester, and carbamates. Specifically, this method displays excellent chemo- and regio-selectivity in carbohydrates, peptides, medicines, and natural products with multiple functionalities.
中文翻译:

通过 C-N 和 C-O 键的光化学裂解对苄基衍生基团进行脱保护
苄基及其衍生物是有机合成中最常用和最广泛使用的保护基团,通常通过传统的过渡金属催化氢解或 Birch 还原来去除。氢解受到官能团相容性以及氢的易燃性和易爆性的限制,而 Birch 还原则受到易燃碱和苛刻条件的限制。在这里,我们报告在温和条件下通过可见光照射很容易去除苄基衍生的保护基团。我们发现激发的酚盐型光催化剂有效地将电子转移到苄基衍生保护基团的苯环上以产生苯基阴离子。苄基 C-N 或 C-O 键然后通过释放苄基自由基被分解裂解。该方法能够有效地脱保护酰胺、芳基胺、醚、酯和氨基甲酸酯上的苄基。具体而言,该方法在碳水化合物、肽、药物和具有多种功能的天然产物中显示出出色的化学和区域选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号