Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: Is there a common intermediate or not?
Journal of Catalysis ( IF 6.5 ) Pub Date : 2015-06-06 15:52:07 Edward L. Kunkes , Felix Studt , Frank Abild-Pedersen , Robert Schlögl , Malte Behrens
Journal of Catalysis ( IF 6.5 ) Pub Date : 2015-06-06 15:52:07 Edward L. Kunkes , Felix Studt , Frank Abild-Pedersen , Robert Schlögl , Malte Behrens
|
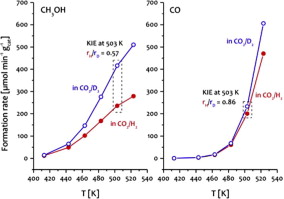
H/D exchange experiments on a Cu/ZnO/Al2O3 catalyst have shown that methanol synthesis and RWGS display a strong thermodynamic isotope effect, which is attributed to differences in the zero-point energy of hydrogenated vs. deuterated species. The effect is larger for methanol synthesis and substantially increases the equilibrium yield in deuterated syngas. In the kinetic regime of CO2 hydrogenation, an inverse kinetic isotope effect of H/D substitution was observed, which is stronger for methanol synthesis than for CO formation suggesting that the two reactions do not share a common intermediate. Similar observations were also made on other catalysts such as Cu/MgO, Cu/SiO2, and Pd/SiO2. In contrast to CO2 hydrogenation, the CO hydrogenation on Cu/ZnO/Al2O3 did not show such a strong kinetic isotope effect indicating that methanol formation from CO2 does not proceed via consecutive reverse water gas shift and CO hydrogenation steps. The inverse KIE is consistent with formate hydrogenation being the rate-determining step of methanol synthesis from CO2. Differences in the extent of product inhibition by water, observed for methanol synthesis and reverse water gas shift indicate that the two reactions proceed on different surface sites in a parallel manner. The consequences for catalyst design for effective methanol synthesis from CO2 are discussed.
中文翻译:

在Cu / ZnO / Al2O3上将CO2加氢为甲醇和CO:是否存在常见的中间体?
在Cu / ZnO / Al 2 O 3催化剂上进行的H / D交换实验表明,甲醇合成和RWGS表现出很强的热力学同位素效应,这归因于氢化物种与氘代物种的零点能量差异。对于甲醇合成,效果更大,并大大提高了氘代合成气的平衡收率。在CO 2氢化的动力学过程中,观察到H / D取代的逆动力学同位素效应,这对于甲醇合成而言比对CO形成更强,表明这两个反应没有共同的中间体。对其他催化剂如Cu / MgO,Cu / SiO 2和Pd / SiO 2也有类似的观察。与CO相反在图2的氢化中,在Cu / ZnO / Al 2 O 3上的CO氢化没有显示出如此强的动力学同位素效应,表明由CO 2形成的甲醇没有通过连续的反向水煤气变换和CO氢化步骤进行。逆KIE与甲酸酯加氢是一致的,甲酸酯加氢是由CO 2合成甲醇的速率确定步骤。观察到的甲醇合成和逆向水煤气变换对水的产物抑制程度的差异表明,这两个反应在平行的不同表面上进行。讨论了由CO 2有效合成甲醇的催化剂设计结果。
更新日期:2015-06-07
中文翻译:

在Cu / ZnO / Al2O3上将CO2加氢为甲醇和CO:是否存在常见的中间体?
在Cu / ZnO / Al 2 O 3催化剂上进行的H / D交换实验表明,甲醇合成和RWGS表现出很强的热力学同位素效应,这归因于氢化物种与氘代物种的零点能量差异。对于甲醇合成,效果更大,并大大提高了氘代合成气的平衡收率。在CO 2氢化的动力学过程中,观察到H / D取代的逆动力学同位素效应,这对于甲醇合成而言比对CO形成更强,表明这两个反应没有共同的中间体。对其他催化剂如Cu / MgO,Cu / SiO 2和Pd / SiO 2也有类似的观察。与CO相反在图2的氢化中,在Cu / ZnO / Al 2 O 3上的CO氢化没有显示出如此强的动力学同位素效应,表明由CO 2形成的甲醇没有通过连续的反向水煤气变换和CO氢化步骤进行。逆KIE与甲酸酯加氢是一致的,甲酸酯加氢是由CO 2合成甲醇的速率确定步骤。观察到的甲醇合成和逆向水煤气变换对水的产物抑制程度的差异表明,这两个反应在平行的不同表面上进行。讨论了由CO 2有效合成甲醇的催化剂设计结果。






























 京公网安备 11010802027423号
京公网安备 11010802027423号