Chemosphere ( IF 8.1 ) Pub Date : 2022-11-18 , DOI: 10.1016/j.chemosphere.2022.137335 Xiaofeng Tang 1 , Wu Xia 2 , Xiaolin Qu 2 , Chaohai Wang 3 , Wenjun Wang 4 , Yuntao Liang 2 , Yuxi Zeng 2 , Weiping Xiong 2 , Min Cheng 2 , Biao Song 2 , Chengyun Zhou 2 , Xiaoying Zhao 5
|
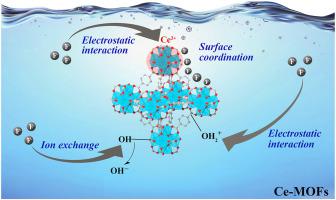
Fluoride in the hydrosphere exceeds the standard, which could be critically hazardous to human health and the natural environment. The adsorption method is a mature and effective way to remove pollutants in water, including fluoride. In this study, we synthesized three kinds of cerium-based metal–organic frameworks (Ce-MOFs) with different structures and properties by modulating the organic ligands (i.e., trimesic acid (BTC), 1,2,4,5-benzenetetracarboxylic acid (PMA), and terephthalic acid (BDC)) via the solvothermal method. The adsorption kinetics of Ce-MOFs on fluoride well fit the pseudo second order model, and their adsorption isotherms also conform to Langmuir isothermal model. The thermodynamic study reveals that the adsorption process is a spontaneous endothermic reaction. The maximum saturated adsorption capacities of Ce-BTC, Ce-PMA, and Ce-BDC are 70.7, 159.6, and 139.5 mg g−1, respectively. Ce-MOFs have stable and excellent adsorption capacity at pH = 3–9. Coexisting anions (Cl−, SO42−, and NO3−) do not affect the performance of Ce-MOFs for fluoride removal. Moreover, Ce-MOFs also show their broad prospect as superior fluoride adsorbents because of their excellent performance and reusability in real water samples. Organic ligands have a remarkable influence on the defluoridation performance of Ce-MOFs. This work will provide a feasible idea for designing MOFs as superiors adsorbents for defluoridation.
中文翻译:

结构-性能相关性指导的铈基金属-有机框架:用于去除水中氟化物的优质吸附剂
水圈中的氟化物超标,对人体健康和自然环境可能造成严重危害。吸附法是一种成熟有效的去除水中污染物(包括氟化物)的方法。在这项研究中,我们通过调节有机配体(即均苯三甲酸(BTC)、1,2,4,5-苯四甲酸)合成了三种具有不同结构和性质的铈基金属有机骨架(Ce-MOFs) (PMA)和对苯二甲酸(BDC))通过溶剂热法。Ce-MOFs对氟化物的吸附动力学符合伪二级模型,吸附等温线也符合Langmuir等温模型。热力学研究表明吸附过程是自发的吸热反应。Ce-BTC、Ce-PMA、-1,分别。Ce-MOFs 在 pH = 3-9 时具有稳定且优异的吸附能力。共存阴离子(Cl -、SO 4 2-和 NO 3 -)不会影响 Ce-MOF 去除氟化物的性能。此外,Ce-MOFs由于其在实际水样中的优异性能和可重复使用性,也显示出其作为优异氟化物吸附剂的广阔前景。有机配体对 Ce-MOFs 的脱氟性能有显着影响。这项工作将为将 MOF 设计为优异的除氟吸附剂提供可行的思路。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号