Environmental Research ( IF 7.7 ) Pub Date : 2022-11-15 , DOI: 10.1016/j.envres.2022.114834 Carmen Mejías 1 , Julia Martín 1 , Juan Luis Santos 1 , Irene Aparicio 1 , Esteban Alonso 1
|
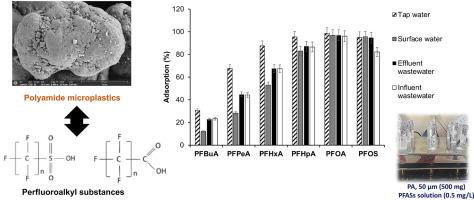
Microplastics (MPs) and perfluoroalkyl substances (PFASs) are two types of pollutants coexisting in the environment. Their co-exposure is a source of increasing concern. MPs present in the natural environment suppose an ideal surface for the sorption of hazardous contaminants. This study investigates the adsorption behaviour of six PFASs on polyamide (PA) MPs. Adsorption experiments under various internal (PA and PFASs dosage, PA particle size) and environmental (pH, ionic strength, dissolved organic matter) factors were carried out. Isotherm results (from 0.1 to 25 mg/L of PFASs) showed that the maximum adsorption capacity of the selected PFASs on the PA was as follows: perfluorooctanesulfonic acid (PFOS, 0.873 mg/g) > perfluorooctanoic acid (0.235 mg/g) > perfluoroheptanoic acid (0.231 mg/g) > perfluorohexanoic acid (0.201 mg/g) > perfluoropentanoic acid (0.192 mg/g) > perfluorobutanoic acid (0.188 mg/g) (pH 5.88, 0% salinity and 0% of dissolved organic matter). The PFOS has more tendency to be sorbed onto PA than perfluorocarboxilic acids. The MP characterization by scanning electron microscopy, X ray diffraction and Fourier transform infrared spectroscopy showed changes in the PA surface after adsorption assays. Pore filling, hydrophobic interactions and hydrogen bonds governed sorption process. The sorption capacity of PFASs was crucially affected by the PA size (from 19.6% to 99.9% for 3 mm and 50 μm particle size, respectively). The process was not significantly influenced by salinity while the dissolved organic matter exerted a negative effect (decrease from 100% to 26% for PFOS in presence of 25 mg/L of humic acid). Finally, adsorption rates of PFASs were quantified in real water matrices (influent and effluent wastewater, surface and tap water samples). The results revealed interactions between PA and PFASs and evidenced the role of PA as a vector to transport PFASs in the aquatic environment.
中文翻译:

全氟烷基物质在聚酰胺微塑料上的吸附:吸附剂的作用和环境因素的影响
微塑料(MPs)和全氟烷基物质(PFASs)是两种共存于环境中的污染物。他们的共同暴露引起了越来越多的关注。存在于自然环境中的 MP 假设是吸附有害污染物的理想表面。本研究调查了六种 PFAS 在聚酰胺 (PA) MP 上的吸附行为。进行了各种内部(PA 和 PFASs 用量、PA 粒径)和环境(pH 值、离子强度、溶解有机物)因素下的吸附实验。等温线结果(从 0.1 到 25 mg/L 的 PFASs)表明,所选 PFASs 在 PA 上的最大吸附容量如下:全氟辛烷磺酸(PFOS,0.873 mg/g)> 全氟辛酸(0.235 mg/g)>全氟庚酸 (0.231 mg/g) > 全氟己酸 (0.201 mg/g) > 全氟戊酸 (0.192 mg/g) > 全氟丁酸 (0.188 mg/g)(pH 值 5.88,0% 盐度和 0% 溶解有机物)。PFOS 比全氟羧酸更容易吸附到 PA 上。通过扫描电子显微镜、X 射线衍射和傅里叶变换红外光谱进行的 MP 表征表明,吸附测定后 PA 表面发生了变化。孔隙填充、疏水相互作用和氢键控制吸附过程。PFAS 的吸附能力主要受 PA 尺寸的影响(对于 3 mm 和 50 μm 的粒径,分别从 19.6% 到 99.9%)。该过程不受盐度的显着影响,而溶解的有机物会产生负面影响(在 25 mg/L 的腐殖酸存在下,PFOS 从 100% 减少到 26%)。最后,PFASs 的吸附率在实际水基质(进水和出水废水、地表水和自来水样品)中进行了量化。结果揭示了 PA 和 PFAS 之间的相互作用,并证明了 PA 作为在水生环境中运输 PFAS 的载体的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号