Chinese Journal of Chemical Engineering Pub Date : 2022-11-18 , DOI: 10.1016/j.cjche.2022.11.002
Xiaohong Zhou , Wenfeng Zhou , Wei Zhuang , Chenjie Zhu , Hanjie Ying , Hongman Zhang
|
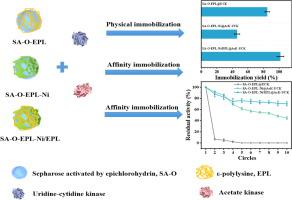
Cytidine 5′-monophosphate (5′-CMP) is an essential nucleotide for additives. In this study, enhanced production of 5′-CMP was realized by the transformation of cytidine using co-immobilized di-enzymes, uridine-cytidine kinase (UCK) and acetate kinase (AcK). The immobilization yield of the enzyme had a clear correlation with the surface charges as zeta potential (ξ). Among them, ε-polylysine-functionalized sepharose (SA-EPL, ξ = 9.31 mV) showed high immobilization yield (78.8%), which was 4.9-fold than that of nitrilotriacetic acid functionalized sepharose (SA-NTA, ξ = −12.6 mV). The residual activity of affinity co-immobilized enzyme (EPL-Ni/EPL@AcK-UCK) was higher than 70.6% after recycled 10 times. Thus, this study provides an effective approach for the production of 5′-CMP with the advantages of low adenosine 5′-triphosphate (ATP) consumption, reduced side reactions, and improved reusability by co-immobilized UCK and AcK on the functionalized Sepharose.
中文翻译:

利用固定在氨基功能化琼脂糖上的双酶生物催化提高胞苷 5'-单磷酸的产量
5'-单磷酸胞苷 (5'-CMP) 是添加剂的必需核苷酸。在这项研究中,通过使用共固定化双酶、尿苷-胞苷激酶 (UCK) 和醋酸激酶 (AcK) 转化胞苷,实现了 5'-CMP 的增强生产。酶的固定化率与作为 zeta 电位 ( ξ ) 的表面电荷有明显的相关性。其中,ε-聚赖氨酸功能化琼脂糖凝胶(SA-EPL,ξ = 9.31 mV)表现出高固定化率(78.8%),是次氮基三乙酸功能化琼脂糖凝胶(SA-NTA,ξ)的 4.9 倍 = −12.6 毫伏)。亲和共固定化酶(EPL-Ni/EPL@AcK-UCK)循环10次后的残留活性高于70.6%。因此,本研究提供了一种生产 5'-CMP 的有效方法,具有 5'-三磷酸腺苷 (ATP) 消耗低、副反应减少以及通过在功能化 Sepharose 上共同固定化 UCK 和 AcK 提高可重用性等优点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号