International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2022-11-17 , DOI: 10.1016/j.ijhydene.2022.10.263
Wu Qin , Ruonan Duan , Congkun Chen , Hengyi Liao , Xianbin Xiao , Zongming Zheng
|
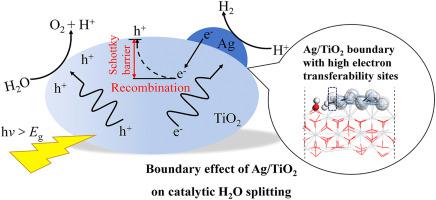
Ag supported on TiO2 reveals advantages in photocatalytic H2O splitting due to its synergistic effects for promoting the e−/h+ separation. The structural and electronic characteristics of Ag/TiO2, as well as adsorption and splitting of H2O on different sites, were investigated employing the density functional theory calculations. The analysis of density of states and electrons densities evidenced the electron transfer and hybrid between Ag and TiO2. The boundary was especially reactive toward the target species being the most active site, and thus electrons could be transferred to the adsorbate through the supported Ag atoms, leading to the generation of Schottky barrier, which inhibited the recombination of photogenerated electron-hole pairs. The O–H bond cleavage with the Ea of 2.18 eV was the rate-determining step for H2 production on the Ag/TiO2 boundary. Fukui functions indicated the outermost Ag cluster site on Ag/TiO2 boundary exhibited high electron transferability and H2 production tendency.
中文翻译:

Ag/TiO2 对催化 H2O 分解产生 H2 的边界效应:理论解释
负载在 TiO 2上的 Ag由于其促进 e - /h +分离的协同效应,显示出在光催化 H 2 O 分解中的优势。采用密度泛函理论计算研究了Ag/TiO 2的结构和电子特性,以及H 2 O在不同位置的吸附和分裂。态密度和电子密度的分析证明了Ag和TiO 2之间的电子转移和杂化. 边界对作为最活跃位点的目标物种具有特别的反应性,因此电子可以通过负载的 Ag 原子转移到吸附物,导致肖特基势垒的产生,从而抑制光生电子-空穴对的重组。E a为 2.18 eV的 O-H 键断裂是 Ag/TiO 2界面上 H 2生成的决速步骤。Fukui函数表明Ag/TiO 2边界上最外层的Ag团簇位点表现出高电子转移能力和H 2生成倾向。

































 京公网安备 11010802027423号
京公网安备 11010802027423号