当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Full Stereocontrol in α-Glycosidation of 3-Deoxy-d-manno-2-octulosonic Acid (Kdo) Using Macrobicyclic Glycosyl Donors
Organic Letters ( IF 4.9 ) Pub Date : 2022-11-18 , DOI: 10.1021/acs.orglett.2c03542 Shogo Hamajima 1 , Naoko Komura 1, 2 , Hide-Nori Tanaka 1, 2 , Akihiro Imamura 1, 2, 3 , Hideharu Ishida 1, 2, 3 , Haruka Noguchi 4 , Tsuyoshi Ichiyanagi 4 , Hiromune Ando 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-11-18 , DOI: 10.1021/acs.orglett.2c03542 Shogo Hamajima 1 , Naoko Komura 1, 2 , Hide-Nori Tanaka 1, 2 , Akihiro Imamura 1, 2, 3 , Hideharu Ishida 1, 2, 3 , Haruka Noguchi 4 , Tsuyoshi Ichiyanagi 4 , Hiromune Ando 1, 2
Affiliation
|
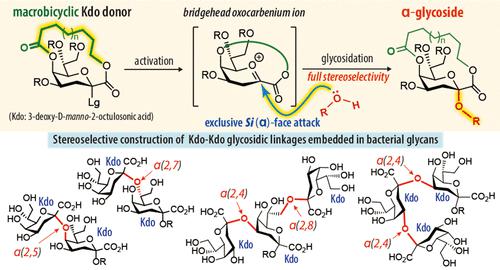
We describe a method for the α-selective glycosidation of 3-deoxy-d-manno-2-octulosonic acid (Kdo) using a macrobicyclic Kdo donor as the precursor of a bridgehead oxocarbenium ion, whose stereoselectivity is not affected by the substrate structure and reaction conditions. Strapping Kdo via tethering in the α-configuration at the C1 and C5 positions completely blocked nucleophilic attack to the β-face of the anomeric center by sterically hindering the bicyclic system, realizing full α-selectivity during glycosidation.
中文翻译:

使用大双环糖基供体对 3-脱氧-d-甘露-2-辛糖酸 (Kdo) 的 α-糖苷化进行完全立体控制
我们描述了一种使用大双环 Kdo 供体作为桥头氧碳鎓离子前体的 3-脱氧-d-甘露糖 -2-octulosonic 酸 (Kdo) 的 α-选择性糖苷化方法,其立体选择性不受底物结构的影响,并且反应条件。通过束缚在 C1 和 C5 位置的 α-构型中束缚 Kdo,通过空间位阻双环系统完全阻断了对异头中心 β-面的亲核攻击,在糖苷化过程中实现了完全的 α-选择性。
更新日期:2022-11-18
中文翻译:

使用大双环糖基供体对 3-脱氧-d-甘露-2-辛糖酸 (Kdo) 的 α-糖苷化进行完全立体控制
我们描述了一种使用大双环 Kdo 供体作为桥头氧碳鎓离子前体的 3-脱氧-d-甘露糖 -2-octulosonic 酸 (Kdo) 的 α-选择性糖苷化方法,其立体选择性不受底物结构的影响,并且反应条件。通过束缚在 C1 和 C5 位置的 α-构型中束缚 Kdo,通过空间位阻双环系统完全阻断了对异头中心 β-面的亲核攻击,在糖苷化过程中实现了完全的 α-选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号