当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiophene-Fused Ladder Boroles with High Antiaromaticity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-05-11 , DOI: 10.1021/ja2019977 Azusa Iida 1 , Shigehiro Yamaguchi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-05-11 , DOI: 10.1021/ja2019977 Azusa Iida 1 , Shigehiro Yamaguchi 1
Affiliation
|
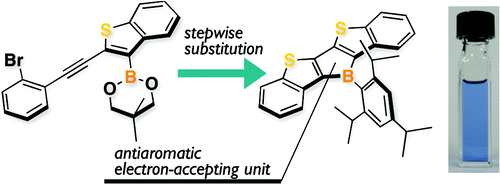
A series of polycyclic thiophene-fused boroles were synthesized on the basis of stepwise substitution reactions from thienylboronic ester precursors. In these ladder-type π-conjugated systems, the thiophene-fused structure enhances the antiaromaticity of the borole ring. This trend is opposite to the conventional understanding that the arene-fused structure decreases the antiaromaticity of the 4π-electron ring skeletons. The ladder boroles exhibited characteristic properties such as long-wavelength absorptions and low reduction potentials.
中文翻译:

具有高抗芳香性的噻吩熔融梯形硼烷
基于噻吩基硼酸酯前体的逐步取代反应,合成了一系列多环噻吩稠合硼化合物。在这些梯型 π 共轭体系中,噻吩稠合结构增强了硼环的抗芳香性。这种趋势与传统的理解相反,即芳烃稠合结构降低了 4π 电子环骨架的反芳香性。梯形硼具有特征性质,例如长波长吸收和低还原电位。
更新日期:2011-05-11
中文翻译:

具有高抗芳香性的噻吩熔融梯形硼烷
基于噻吩基硼酸酯前体的逐步取代反应,合成了一系列多环噻吩稠合硼化合物。在这些梯型 π 共轭体系中,噻吩稠合结构增强了硼环的抗芳香性。这种趋势与传统的理解相反,即芳烃稠合结构降低了 4π 电子环骨架的反芳香性。梯形硼具有特征性质,例如长波长吸收和低还原电位。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号