Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2022-11-15 , DOI: 10.1016/j.apsb.2022.10.022 Yub Raj Neupane 1, 2 , Harish K Handral 3 , Syed Abdullah Alkaff 4 , Wei Heng Chng 1, 5 , Gopalakrishnan Venkatesan 1, 6 , Chenyuan Huang 7, 8, 9 , Choon Keong Lee 1 , Jiong-Wei Wang 7, 8, 9, 10 , Gopu Sriram 11 , Rhonnie Austria Dienzo 12 , Wen Feng Lu 3 , Yusuf Ali 12, 13 , Bertrand Czarny 4, 12 , Giorgia Pastorin 1, 5, 14
|
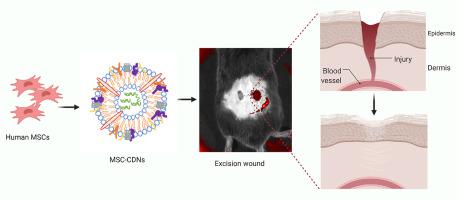
Wound healing is a dynamic process that involves a series of molecular and cellular events aimed at replacing devitalized and missing cellular components and/or tissue layers. Recently, extracellular vesicles (EVs), naturally cell-secreted lipid membrane-bound vesicles laden with biological cargos including proteins, lipids, and nucleic acids, have drawn wide attention due to their ability to promote wound healing and tissue regeneration. However, current exploitation of EVs as therapeutic agents is limited by their low isolation yields and tedious isolation processes. To circumvent these challenges, bioinspired cell-derived nanovesicles (CDNs) that mimic EVs were obtained by shearing mesenchymal stem cells (MSCs) through membranes with different pore sizes. Physical characterisations and high-throughput proteomics confirmed that MSC-CDNs mimicked MSC-EVs. Moreover, these MSC-CDNs were efficiently uptaken by human dermal fibroblasts and demonstrated a dose-dependent activation of MAPK signalling pathway, resulting in enhancement of cell proliferation, cell migration, secretion of growth factors and extracellular matrix proteins, which all promoted tissue regeneration. Of note, MSC-CDNs enhanced angiogenesis in human dermal microvascular endothelial cells in a 3D PEG-fibrin scaffold and animal model, accelerating wound healing in vitro and in vivo. These findings suggest that MSC-CDNs could replace both whole cells and EVs in promoting wound healing and tissue regeneration.
中文翻译:

来自间充质干细胞的细胞衍生纳米囊泡作为伤口愈合中的细胞外囊泡模拟物
伤口愈合是一个动态过程,涉及一系列旨在替换失活和缺失的细胞成分和/或组织层的分子和细胞事件。最近,细胞外囊泡(EVs),自然细胞分泌的脂质膜结合囊泡,载有包括蛋白质、脂质和核酸在内的生物物质,由于其促进伤口愈合和组织再生的能力而受到广泛关注。然而,目前将 EV 作为治疗剂的开发受到其低分离产量和繁琐的分离过程的限制。为了规避这些挑战,通过剪切间充质干细胞 (MSC) 穿过具有不同孔径的膜来获得模拟 EV 的仿生细胞衍生纳米囊泡 (CDN)。物理特征和高通量蛋白质组学证实 MSC-CDN 模仿了 MSC-EV。此外,这些 MSC-CDN 被人真皮成纤维细胞有效摄取,并显示出 MAPK 信号通路的剂量依赖性激活,从而促进细胞增殖、细胞迁移、生长因子和细胞外基质蛋白的分泌,这些都促进了组织再生。值得注意的是,MSC-CDNs 在 3D PEG-纤维蛋白支架和动物模型中增强了人真皮微血管内皮细胞的血管生成,加速了伤口愈合 生长因子和细胞外基质蛋白的分泌,它们都促进组织再生。值得注意的是,MSC-CDNs 在 3D PEG-纤维蛋白支架和动物模型中增强了人真皮微血管内皮细胞的血管生成,加速了伤口愈合 生长因子和细胞外基质蛋白的分泌,它们都促进组织再生。值得注意的是,MSC-CDNs 在 3D PEG-纤维蛋白支架和动物模型中增强了人真皮微血管内皮细胞的血管生成,加速了伤口愈合体外和体内。这些发现表明,MSC-CDN 可以替代全细胞和 EV,促进伤口愈合和组织再生。































 京公网安备 11010802027423号
京公网安备 11010802027423号