当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward a Practical Catalyst for Convenient Deaminative Hydrogenation of Amides under Mild Conditions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-11-16 , DOI: 10.1021/acssuschemeng.2c05802
Laurynas Gausas 1, 2 , Ralf Jackstell 3 , Troels Skrydstrup 1, 2 , Matthias Beller 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-11-16 , DOI: 10.1021/acssuschemeng.2c05802
Laurynas Gausas 1, 2 , Ralf Jackstell 3 , Troels Skrydstrup 1, 2 , Matthias Beller 3
Affiliation
|
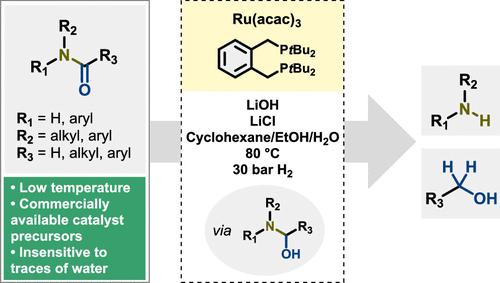
Amide bond reduction is a versatile transformation offering access to various alcohols and amines that could be used as valuable precursors in the chemical and pharmaceutical industries, e.g., for manufacturing plastics, textiles, dyes, agrochemicals, etc. Over the last two decades, catalytic amide hydrogenation employing homogeneous catalysis has gained more attention due to the atom efficiency and low environmental impact of this transformation. Owing to the inherent strength of amide bonds, amide hydrogenation procedures often involve high temperatures and pressures, which is why efforts are being channeled to finding protocols with lower-energy input. Here, we report a mild amide hydrogenation method involving commercially available precursors Ru(acac)3 and 1,2-bis(di-tert-butylphosphinomethyl)benzene (L4), which under basic conditions, at 80 °C and under 30 bar of H2, can selectively hydrogenate a series of 2°-benzamides to anilines and alcohols with yields of 36–98% and 29–92%, respectively. Additionally, 1°- and 3°-amides proved to be appropriate substrates; however, low to moderate yields were obtained. The catalyst is believed to operate via an inner-sphere mechanism with a hemiaminal being the likely intermediate during the hydrogenation process.
中文翻译:

开发一种在温和条件下方便地使酰胺脱氨氢化的实用催化剂
酰胺键还原是一种多功能转化,提供了获取各种醇和胺的途径,这些醇和胺可用作化学和制药行业中有价值的前体,例如,用于制造塑料、纺织品、染料、农用化学品等。在过去的二十年中,催化酰胺由于这种转化的原子效率和低环境影响,采用均相催化的氢化得到了更多的关注。由于酰胺键的固有强度,酰胺氢化过程通常涉及高温和高压,这就是为什么人们正在努力寻找能量输入较低的方案。在这里,我们报告了一种温和的酰胺氢化方法,涉及市售前体 Ru(acac) 3和 1,2-bis(di- tert-丁基膦甲基)苯 ( L4 ), 在碱性条件下, 80 °C 和 30 bar H 2下, 可以选择性地将一系列 2°-苯甲酰胺加氢为苯胺和醇, 收率为 36–98% 和 29–92 %, 分别。此外,1°- 和 3°- 酰胺被证明是合适的底物;然而,获得了低到中等的产量。据信该催化剂通过内球机制运行,半缩醛胺可能是氢化过程中的中间体。
更新日期:2022-11-16
中文翻译:

开发一种在温和条件下方便地使酰胺脱氨氢化的实用催化剂
酰胺键还原是一种多功能转化,提供了获取各种醇和胺的途径,这些醇和胺可用作化学和制药行业中有价值的前体,例如,用于制造塑料、纺织品、染料、农用化学品等。在过去的二十年中,催化酰胺由于这种转化的原子效率和低环境影响,采用均相催化的氢化得到了更多的关注。由于酰胺键的固有强度,酰胺氢化过程通常涉及高温和高压,这就是为什么人们正在努力寻找能量输入较低的方案。在这里,我们报告了一种温和的酰胺氢化方法,涉及市售前体 Ru(acac) 3和 1,2-bis(di- tert-丁基膦甲基)苯 ( L4 ), 在碱性条件下, 80 °C 和 30 bar H 2下, 可以选择性地将一系列 2°-苯甲酰胺加氢为苯胺和醇, 收率为 36–98% 和 29–92 %, 分别。此外,1°- 和 3°- 酰胺被证明是合适的底物;然而,获得了低到中等的产量。据信该催化剂通过内球机制运行,半缩醛胺可能是氢化过程中的中间体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号