当前位置:
X-MOL 学术
›
Dev. Growth Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vitro and in vivo gene transfer in the cloudy catshark Scyliorhinus torazame
Development, Growth & Differentiation ( IF 1.7 ) Pub Date : 2022-11-14 , DOI: 10.1111/dgd.12824 Chika Fujimori 1 , Chie Umatani 2 , Misaki Chimura 3 , Shigeho Ijiri 3 , Hisanori Bando 4 , Susumu Hyodo 1 , Shinji Kanda 1
Development, Growth & Differentiation ( IF 1.7 ) Pub Date : 2022-11-14 , DOI: 10.1111/dgd.12824 Chika Fujimori 1 , Chie Umatani 2 , Misaki Chimura 3 , Shigeho Ijiri 3 , Hisanori Bando 4 , Susumu Hyodo 1 , Shinji Kanda 1
Affiliation
|
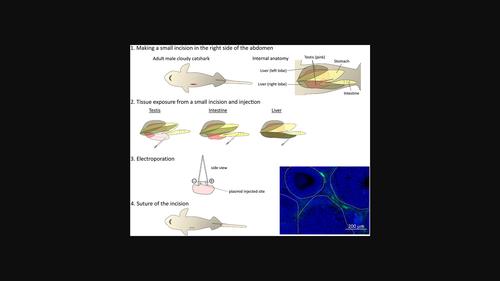
Cartilaginous fishes have various unique physiological features such as a cartilaginous skeleton and a urea-based osmoregulation strategy for adaptation to their marine environment. Also, because they are a sister group of bony vertebrates, understanding their unique features is important from an evolutionary perspective. However, genetic engineering based on gene functions as well as cellular behavior has not been effectively utilized in cartilaginous fishes. This is partly because their reproductive strategy involves internal fertilization, which results in difficulty in microinjection into fertilized eggs at the early developmental stage. Here, to identify efficient gene transfer methods in cartilaginous fishes, we examined the effects of various methods both in vitro and in vivo using the cloudy catshark, a candidate model cartilaginous fish species. In all methods, green fluorescent protein (GFP) expression was used to evaluate exogenous gene transfer. First, we examined gene transfer into primary cultured cells from cloudy catshark embryos by lipofection, polyethylenimine (PEI) transfection, adenovirus infection, baculovirus infection, and electroporation. Among the methods tested, lipofection, electroporation, and baculovirus infection enabled the successful transfer of exogenous genes into primary cultured cells. We then attempted in vivo transfection into cloudy catshark embryos by electroporation and baculovirus infection. Although baculovirus-injected groups did not show GFP fluorescence, electroporation successfully introduced GFP into muscle cells. Furthermore, we succeeded in GFP transfer into adult tissues by electroporation. The in vitro and in vivo gene transfer methods that worked in this study may open ways for genetic manipulation including knockout experiments and cellular lineage analysis in cartilaginous fishes.
中文翻译:

云猫鲨 Scyliorhinus torazame 的体外和体内基因转移
软骨鱼类具有各种独特的生理特征,例如软骨骨骼和基于尿素的渗透调节策略以适应其海洋环境。此外,由于它们是骨脊椎动物的姐妹群,因此从进化的角度了解它们的独特特征也很重要。然而,基于基因功能和细胞行为的基因工程尚未在软骨鱼类中得到有效利用。这部分是因为它们的繁殖策略涉及内部受精,这导致难以在早期发育阶段显微注射到受精卵中。在这里,为了确定软骨鱼类中有效的基因转移方法,我们使用多云猫鲨检查了体外和体内各种方法的效果,候选模型软骨鱼类。在所有方法中,绿色荧光蛋白 (GFP) 表达用于评估外源基因转移。首先,我们通过脂质转染、聚乙烯亚胺 (PEI) 转染、腺病毒感染、杆状病毒感染和电穿孔检查了基因从多云猫鲨胚胎转移到原代培养细胞中的情况。在测试的方法中,脂肪转染、电穿孔和杆状病毒感染使外源基因成功转移到原代培养细胞中。然后我们尝试通过电穿孔和杆状病毒感染将体内转染到多云猫鲨胚胎中。虽然杆状病毒注射组未显示 GFP 荧光,但电穿孔成功地将 GFP 引入肌肉细胞。此外,我们通过电穿孔成功地将 GFP 转移到成人组织中。
更新日期:2022-11-14
中文翻译:

云猫鲨 Scyliorhinus torazame 的体外和体内基因转移
软骨鱼类具有各种独特的生理特征,例如软骨骨骼和基于尿素的渗透调节策略以适应其海洋环境。此外,由于它们是骨脊椎动物的姐妹群,因此从进化的角度了解它们的独特特征也很重要。然而,基于基因功能和细胞行为的基因工程尚未在软骨鱼类中得到有效利用。这部分是因为它们的繁殖策略涉及内部受精,这导致难以在早期发育阶段显微注射到受精卵中。在这里,为了确定软骨鱼类中有效的基因转移方法,我们使用多云猫鲨检查了体外和体内各种方法的效果,候选模型软骨鱼类。在所有方法中,绿色荧光蛋白 (GFP) 表达用于评估外源基因转移。首先,我们通过脂质转染、聚乙烯亚胺 (PEI) 转染、腺病毒感染、杆状病毒感染和电穿孔检查了基因从多云猫鲨胚胎转移到原代培养细胞中的情况。在测试的方法中,脂肪转染、电穿孔和杆状病毒感染使外源基因成功转移到原代培养细胞中。然后我们尝试通过电穿孔和杆状病毒感染将体内转染到多云猫鲨胚胎中。虽然杆状病毒注射组未显示 GFP 荧光,但电穿孔成功地将 GFP 引入肌肉细胞。此外,我们通过电穿孔成功地将 GFP 转移到成人组织中。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号