当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-Phase Synthesis of C-Terminus Cysteine Peptide Acids
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.oprd.2c00321 Sinenhlanhla N. Mthembu 1, 2 , Amit Chakraborty 1 , Ralph Schönleber 3 , Fernando Albericio 1, 4, 5 , Beatriz G. de la Torre 1, 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.oprd.2c00321 Sinenhlanhla N. Mthembu 1, 2 , Amit Chakraborty 1 , Ralph Schönleber 3 , Fernando Albericio 1, 4, 5 , Beatriz G. de la Torre 1, 2
Affiliation
|
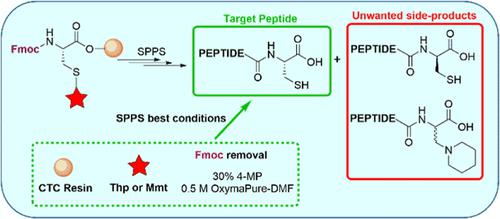
Cysteine (Cys) is a key amino acid in many therapeutic peptides. For research and industrial purposes, solid-phase peptide synthesis is the method of choice for the preparation of most peptides. The solid-phase synthesis of C-terminal Cys peptide acids is problematic because it is accompanied by a side reaction, namely, the abstraction of α-H from the Cys residue, which leads to the formation of three side products: the epimer and two N-piperidinyl-Ala epimer peptides. Here, we used a chlorotrityl chloride resin to conduct a rational and in-depth study of this side reaction. The following variables were examined: removal of the fluorenylmethoxycarbonyl (Fmoc) group by different bases, the presence or absence of an acid rectifier for buffering the base, and thiol side-chain protection. In conclusion, the use of Fmoc-Cys protected with tetrahydropyran (Thp) and 4-methoxytrityl (Mmt) along with 30% 4-methylpiperidine in 0.5 M OxymaPure-DMF for Fmoc removal assures minimization of the side reaction, as demonstrated in a model peptide and confirmed for the elongation of somatostatin.
中文翻译:

C-末端半胱氨酸肽酸的固相合成
半胱氨酸 (Cys) 是许多治疗性肽中的关键氨基酸。对于研究和工业目的,固相肽合成是制备大多数肽的首选方法。C 端 Cys 肽酸的固相合成是有问题的,因为它伴随着副反应,即从 Cys 残基中提取 α-H,这导致三种副产物的形成:差向异构体和两个N-哌啶基丙氨酸差向异构肽。在这里,我们使用氯三苯甲基氯树脂对该副反应进行了合理深入的研究。检查了以下变量:通过不同碱基去除芴基甲氧基羰基 (Fmoc)、是否存在用于缓冲碱基的酸整流剂以及硫醇侧链保护。总之,使用四氢吡喃 (Thp) 和 4-甲氧基三苯甲基 (Mmt) 保护的 Fmoc-Cys 以及 30% 4-甲基哌啶在 0.5 M OxymaPure-DMF 中去除 Fmoc 可确保将副反应降至最低,如模型所示肽并证实了生长抑素的延长。
更新日期:2022-11-15
中文翻译:

C-末端半胱氨酸肽酸的固相合成
半胱氨酸 (Cys) 是许多治疗性肽中的关键氨基酸。对于研究和工业目的,固相肽合成是制备大多数肽的首选方法。C 端 Cys 肽酸的固相合成是有问题的,因为它伴随着副反应,即从 Cys 残基中提取 α-H,这导致三种副产物的形成:差向异构体和两个N-哌啶基丙氨酸差向异构肽。在这里,我们使用氯三苯甲基氯树脂对该副反应进行了合理深入的研究。检查了以下变量:通过不同碱基去除芴基甲氧基羰基 (Fmoc)、是否存在用于缓冲碱基的酸整流剂以及硫醇侧链保护。总之,使用四氢吡喃 (Thp) 和 4-甲氧基三苯甲基 (Mmt) 保护的 Fmoc-Cys 以及 30% 4-甲基哌啶在 0.5 M OxymaPure-DMF 中去除 Fmoc 可确保将副反应降至最低,如模型所示肽并证实了生长抑素的延长。






























 京公网安备 11010802027423号
京公网安备 11010802027423号