Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2022-11-15 , DOI: 10.1016/j.xcrp.2022.101153 Rui Gao , Ningyi Zhong , Linjing Tong , Xiaoxue Kou , Wei Huang , Huangsheng Yang , Siming Huang , Jiayi Wu , Guosheng Chen , Gangfeng Ouyang
|
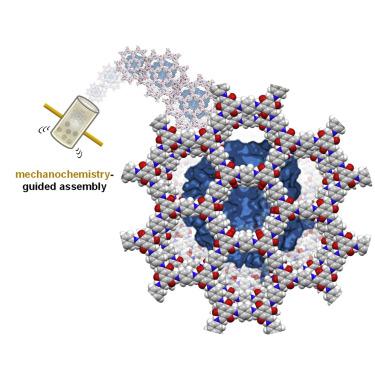
Structural stabilization of enzymes by porous frameworks is an emerging strategy in biotechnology that may accelerate large-scale industrial application of biocatalysts. Herein, we stabilize an enzyme by wrapping it with covalent organic frameworks in situ using a mild mechanochemistry-guided assembly. This mechanochemical strategy circumvents the harsh conditions often necessary for covalent organic frameworks’ crystallization, and hence avoids enzyme denaturation during the de novo encapsulation. The covalent-linked topology and tailorable large pore channels of the covalent organic framework host not only stabilize enzymes, but also outperform the well-explored enzyme-zeolite imidazole framework counterparts in terms of catalytic efficiency, functional diversity, and structural stability. This work offers a greener and convenient mechanochemistry-guided assembly strategy, enabling template-free enzyme encapsulation across multiple enzyme and covalent organic framework pore structures.
中文翻译:

机械化学引导的网状组装,用于稳定具有共价有机框架的酶
通过多孔框架稳定酶的结构是生物技术中的一种新兴策略,可以加速生物催化剂的大规模工业应用。在这里,我们使用温和的机械化学引导组装,通过原位用共价有机框架包裹酶来稳定酶。这种机械化学策略规避了共价有机框架结晶通常需要的苛刻条件,因此避免了从头开始过程中的酶变性封装。共价有机框架主体的共价连接拓扑结构和可定制的大孔通道不仅可以稳定酶,而且在催化效率、功能多样性和结构稳定性方面优于已充分探索的酶-沸石咪唑框架对应物。这项工作提供了一种更环保、更方便的机械化学引导组装策略,实现了跨多种酶和共价有机骨架孔结构的无模板酶封装。































 京公网安备 11010802027423号
京公网安备 11010802027423号