Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2022-11-15 , DOI: 10.1016/j.bbapap.2022.140869
Asaf Shemesh 1 , Hiba Ghareeb 2 , Raviv Dharan 2 , Yael Levi-Kalisman 3 , Norman Metanis 2 , Israel Ringel 4 , Uri Raviv 1
|
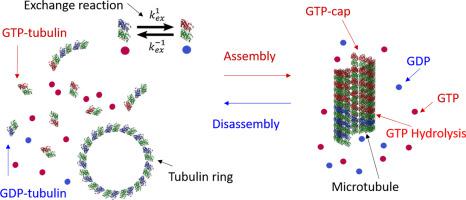
We investigated how the self-association of isolated tubulin dimers affects the rate of GTP hydrolysis and the equilibrium of nucleotide exchange. Both reactions are relevant for microtubule (MT) dynamics. We used HPLC to determine the concentrations of GDP and GTP and thereby the GTPase activity of SEC-eluted tubulin dimers in assembly buffer solution, free of glycerol and tubulin aggregates. When GTP hydrolysis was negligible, the nucleotide exchange mechanism was studied by determining the concentrations of tubulin-free and tubulin-bound GTP and GDP. We observed no GTP hydrolysis below the critical conditions for MT assembly (either below the critical tubulin concentration and/or at low temperature), despite the assembly of tubulin 1D curved oligomers and single-rings, showing that their assembly did not involve GTP hydrolysis. Under conditions enabling spontaneous slow MT assembly, a slow pseudo-first-order GTP hydrolysis kinetics was detected, limited by the rate of MT assembly. Cryo-TEM images showed that GTP-tubulin 1D oligomers were curved also at 36 °C. Nucleotide exchange depended on the total tubulin concentration and the molar ratio between tubulin-free GDP and GTP. We used a thermodynamic model of isodesmic tubulin self-association, terminated by the formation of tubulin single-rings to determine the molar fractions of dimers with exposed and buried nucleotide exchangeable sites (E-sites). Our analysis shows that the GDP to GTP exchange reaction equilibrium constant was an order-of-magnitude larger for tubulin dimers with exposed E-sites than for assembled dimers with buried E-sites. This conclusion may have implications on the dynamics at the tip of the MT plus end.
中文翻译:

微管蛋白自结合对 GTP 水解和核苷酸交换反应的影响
我们研究了分离的微管蛋白二聚体的自缔合如何影响 GTP 水解速率和核苷酸交换的平衡。这两种反应都与微管 (MT) 动力学相关。我们使用 HPLC 来确定 GDP 和 GTP 的浓度,从而确定组装缓冲溶液中 SEC 洗脱的微管蛋白二聚体的 GTPase 活性,不含甘油和微管蛋白聚集体。当 GTP 水解可忽略不计时,通过确定无微管蛋白和微管蛋白结合的 GTP 和 GDP 的浓度来研究核苷酸交换机制。我们观察到在 MT 组装的临界条件下(低于临界微管蛋白浓度和/或低温)没有 GTP 水解,尽管微管蛋白 1D 弯曲低聚物和单环组装,表明它们的组装不涉及 GTP 水解。在允许自发缓慢 MT 组装的条件下,检测到缓慢的伪一级 GTP 水解动力学,受 MT 组装速率的限制。Cryo-TEM 图像显示 GTP-微管蛋白 1D 寡聚体也在 36°C 下弯曲。核苷酸交换取决于总微管蛋白浓度和无微管蛋白 GDP 与 GTP 之间的摩尔比。我们使用等渗微管蛋白自缔合的热力学模型,终止于微管蛋白单环的形成,以确定具有暴露和掩埋的核苷酸可交换位点(E 位点)的二聚体的摩尔分数。我们的分析表明,具有暴露 E 位点的微管蛋白二聚体的 GDP 到 GTP 交换反应平衡常数比具有掩埋 E 位点的组装二聚体大一个数量级。

































 京公网安备 11010802027423号
京公网安备 11010802027423号