当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbonylative Co- and Terpolymerizations of 10-Undecen-1-ol: A Route to Polyketoesters with Tunable Compositions
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-15 , DOI: 10.1021/acscatal.2c03984 Shao-Yu Lo 1 , Carlton P. Folster 1 , Robin P. Harkins 1 , Ryan J. Anderson 1 , Yu-Ling Lien 1 , Hsin-Chun Chiu 1 , Alex E. Carpenter 1 , Ian A. Tonks 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-15 , DOI: 10.1021/acscatal.2c03984 Shao-Yu Lo 1 , Carlton P. Folster 1 , Robin P. Harkins 1 , Ryan J. Anderson 1 , Yu-Ling Lien 1 , Hsin-Chun Chiu 1 , Alex E. Carpenter 1 , Ian A. Tonks 1
Affiliation
|
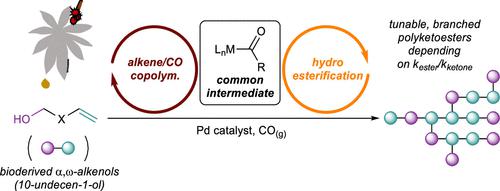
A strategy to synthesize branched polyketoesters from the carbonylative polymerization of bifunctional α,ω-alkenols such as 10-undecen-1-ol is presented. This strategy hinges on the competitive application of two related catalytic manifolds, alternating alkene/CO copolymerization, and alkene hydroesterification, which share a common metal acyl intermediate. Small molecule model studies of cationic Pd-catalyzed alkene carbonylation in the presence of alcohols demonstrate that the relative rates of ketone formation (through alternating alkene/CO insertion) and ester formation (through metal acyl alcoholysis) can be tuned across a wide range through judicious bis(phosphine) ligand design. Carbonylative polymerization of 10-undecen-1-ol with a (dppp(3,5-CF3)4)Pd(OTs)2 catalyst (dppp(3,5-CF3)4 = 1,3-bis[bis[3,5-bis(trifluoromethyl)phenyl]-phosphino]propane) led to the formation of high molecular weight polyketoesters with intermediate dispersity (Mn > 20,000 g/mol, D̵ = 2.6) and a ketone/ester microstructure ratio of approximately 1:2. In these polymerization reactions, deploying electron-deficient bis(phosphines) to suppress deleterious alkene isomerization was the key to accessing the high molecular weight polymer. Further, terpolymerization reactions of 1-hexene/10-undecen-1-ol/CO or 1-fluoro-10-undecene/10-undecen-1-ol/CO by (dppp(3,5-CF3)4)Pd(OTs)2 were also successful. This proof of concept polymerization unlocks access to tunable polymer microstructures without extensive postpolymerization treatment.
中文翻译:

10-十一碳烯-1-醇的羰基共聚和三元聚合:制备具有可调成分的聚酮酯的途径
提出了一种通过双功能 α,ω-烯醇(例如 10-十一碳烯-1-醇)的羰基化聚合合成支化聚酮酯的策略。该策略取决于两个相关催化歧管的竞争应用,交替烯烃/CO 共聚和烯烃加氢酯化,它们共享一个共同的金属酰基中间体。在醇存在下阳离子 Pd 催化的烯烃羰基化的小分子模型研究表明,酮形成(通过交替的烯烃/CO 插入)和酯形成(通过金属酰基醇解)的相对速率可以通过明智的调整在很宽的范围内双(膦)配体设计。10-十一碳烯-1-醇与 (dppp(3,5-CF 3 ) 4 )Pd(OTs) 2的羰基聚合反应催化剂 (dppp(3,5-CF 3 ) 4 = 1,3-bis[bis[3,5-bis(trifluoromethyl)phenyl]-phosphino]propane) 导致形成具有中等分散性的高分子量聚酮酯 ( M n > 20,000 g/mol, D̵ = 2.6) 和酮/酯微结构比约为 1:2。在这些聚合反应中,利用缺电子双(膦)来抑制有害的烯烃异构化是获得高分子量聚合物的关键。此外,1-己烯/10-十一碳烯-1-醇/CO或1-氟-10-十一碳烯/10-十一碳烯-1-醇/CO通过(dppp(3,5-CF 3 ) 4 )Pd的三聚反应(OT) 2也很成功。这种概念聚合证明无需大量的聚合后处理即可获得可调谐的聚合物微观结构。
更新日期:2022-11-15
中文翻译:

10-十一碳烯-1-醇的羰基共聚和三元聚合:制备具有可调成分的聚酮酯的途径
提出了一种通过双功能 α,ω-烯醇(例如 10-十一碳烯-1-醇)的羰基化聚合合成支化聚酮酯的策略。该策略取决于两个相关催化歧管的竞争应用,交替烯烃/CO 共聚和烯烃加氢酯化,它们共享一个共同的金属酰基中间体。在醇存在下阳离子 Pd 催化的烯烃羰基化的小分子模型研究表明,酮形成(通过交替的烯烃/CO 插入)和酯形成(通过金属酰基醇解)的相对速率可以通过明智的调整在很宽的范围内双(膦)配体设计。10-十一碳烯-1-醇与 (dppp(3,5-CF 3 ) 4 )Pd(OTs) 2的羰基聚合反应催化剂 (dppp(3,5-CF 3 ) 4 = 1,3-bis[bis[3,5-bis(trifluoromethyl)phenyl]-phosphino]propane) 导致形成具有中等分散性的高分子量聚酮酯 ( M n > 20,000 g/mol, D̵ = 2.6) 和酮/酯微结构比约为 1:2。在这些聚合反应中,利用缺电子双(膦)来抑制有害的烯烃异构化是获得高分子量聚合物的关键。此外,1-己烯/10-十一碳烯-1-醇/CO或1-氟-10-十一碳烯/10-十一碳烯-1-醇/CO通过(dppp(3,5-CF 3 ) 4 )Pd的三聚反应(OT) 2也很成功。这种概念聚合证明无需大量的聚合后处理即可获得可调谐的聚合物微观结构。







































 京公网安备 11010802027423号
京公网安备 11010802027423号