当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent updates on 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-11-13 , DOI: 10.1002/ardp.202200479 Gaoli Dong 1 , Yingchun Jiang 2 , Feng Zhang 1 , Fengyun Zhu 3 , Junna Liu 4 , Zhi Xu 4
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-11-13 , DOI: 10.1002/ardp.202200479 Gaoli Dong 1 , Yingchun Jiang 2 , Feng Zhang 1 , Fengyun Zhu 3 , Junna Liu 4 , Zhi Xu 4
Affiliation
|
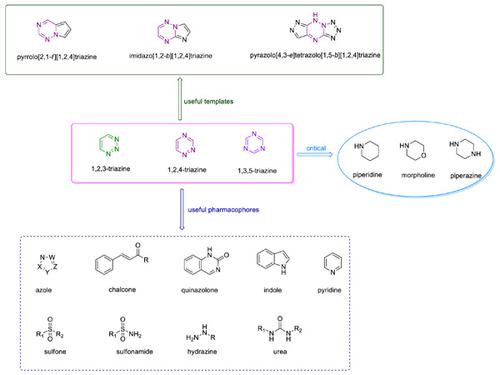
Cancer is one of the leading causes of death across the world, and the prevalence and mortality rates of cancer will continue to grow. Chemotherapeutics play a critical role in cancer therapy, but drug resistance and side effects are major hurdles to effective treatment, evoking an immediate need for the discovery of new anticancer agents. Triazines including 1,2,3-, 1,2,4-, and 1,3,5-triazine have occupied a propitious place in drug design and development due to their excellent pharmacological profiles. Mechanistically, triazine derivatives could interfere with various signaling pathways to induce cancer cell death. Hence, triazine derivatives possess potential in vitro and in vivo efficacy against diverse cancers. In particular, triazine hybrids are able to overcome drug resistance and reduce side effects. Moreover, several triazine hybrids such as brivanib (indole-containing pyrrolo[2,1-f][1,2,4]triazine), gedatolisib (1,3,5-triazine-urea hybrid), and enasidenib (1,3,5-triazine-pyridine hybrid) have already been available in the market. Accordingly, triazine hybrids are useful scaffolds for the discovery of novel anticancer chemotherapeutics. This review focuses on the anticancer activity of 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids, together with the structure–activity relationships and mechanisms of action developed from 2017 to the present. The enriched structure–activity relationships may be useful for further rational drug development of triazine hybrids as potential clinical candidates.
中文翻译:

1,2,3-、1,2,4- 和 1,3,5-三嗪杂化物(2017 年至今)的最新更新:抗癌活性、构效关系和作用机制
癌症是全世界死亡的主要原因之一,癌症的患病率和死亡率将继续增长。化学疗法在癌症治疗中起着关键作用,但耐药性和副作用是有效治疗的主要障碍,因此迫切需要发现新的抗癌药物。包括 1,2,3-、1,2,4- 和 1,3,5-三嗪在内的三嗪由于其出色的药理学特性,在药物设计和开发中占有重要地位。从机制上讲,三嗪衍生物可以干扰各种信号通路以诱导癌细胞死亡。因此,三嗪衍生物具有潜在的体外和体内抗多种癌症的功效。特别是,三嗪杂化物能够克服耐药性并减少副作用。而且,f ][1,2,4]三嗪)、gedatolisib(1,3,5-三嗪-尿素杂化物)和enasidenib(1,3,5-三嗪-吡啶杂化物)已经上市。因此,三嗪杂化物是发现新型抗癌化疗药物的有用支架。本综述侧重于 1,2,3-、1,2,4- 和 1,3,5-三嗪杂化物的抗癌活性,以及从 2017 年至今发展起来的构效关系和作用机制。丰富的构效关系可能有助于三嗪杂化物作为潜在临床候选药物的进一步合理药物开发。
更新日期:2022-11-13
中文翻译:

1,2,3-、1,2,4- 和 1,3,5-三嗪杂化物(2017 年至今)的最新更新:抗癌活性、构效关系和作用机制
癌症是全世界死亡的主要原因之一,癌症的患病率和死亡率将继续增长。化学疗法在癌症治疗中起着关键作用,但耐药性和副作用是有效治疗的主要障碍,因此迫切需要发现新的抗癌药物。包括 1,2,3-、1,2,4- 和 1,3,5-三嗪在内的三嗪由于其出色的药理学特性,在药物设计和开发中占有重要地位。从机制上讲,三嗪衍生物可以干扰各种信号通路以诱导癌细胞死亡。因此,三嗪衍生物具有潜在的体外和体内抗多种癌症的功效。特别是,三嗪杂化物能够克服耐药性并减少副作用。而且,f ][1,2,4]三嗪)、gedatolisib(1,3,5-三嗪-尿素杂化物)和enasidenib(1,3,5-三嗪-吡啶杂化物)已经上市。因此,三嗪杂化物是发现新型抗癌化疗药物的有用支架。本综述侧重于 1,2,3-、1,2,4- 和 1,3,5-三嗪杂化物的抗癌活性,以及从 2017 年至今发展起来的构效关系和作用机制。丰富的构效关系可能有助于三嗪杂化物作为潜在临床候选药物的进一步合理药物开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号