当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PtI4-catalyzed oxidative and hydrogenative dearomative [3 + 2] cycloaddition of 1H-indole N-tethered o-alkynylbenzaldehydes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-14 , DOI: 10.1039/d2qo01520j Dandan Shang 1 , Rui Hu 1 , Qing Bao 1 , Jichao Chen 1 , Lei Yu 2 , Philip Wai Hong Chan 2 , Weidong Rao 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-14 , DOI: 10.1039/d2qo01520j Dandan Shang 1 , Rui Hu 1 , Qing Bao 1 , Jichao Chen 1 , Lei Yu 2 , Philip Wai Hong Chan 2 , Weidong Rao 1
Affiliation
|
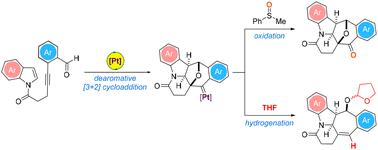
A synthetic method for the chemodivergent assembly of a diverse range of highly functionalized and architecturally challenging cyclohepta[b]indolines which relies on PtI4-catalyzed oxidative and hydrogenative dearomative [3 + 2] cycloaddition of 1H-indole N-tethered o-alkynylbenzaldehydes in a single operation is described. For the synthesis of a key structural feature that is found in a myriad of bioactive natural products and pharmaceutical compounds, the proposed cascade chemodivergent process delineates the first example of an in situ formed Pt-bound benzopyrylium intermediate that participates in [3 + 2] cycloaddition with the C(2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(3) bond of an aromatic N-heterocycle as the 2π cycloaddition partner with exclusive exo-selectivity in a highly efficient manner. It also offers a unique instance of the resulting metallocarbene species generated in this manner undergoing either oxidation by phenylmethylsulfoxide or reduction by THF, with the solvent as both the hydride and alkyl reagent source. The chemodivergent protocol exhibits exceptional functional group tolerance and is amenable to the late-stage modification of a series of structurally complex bioactive natural products and drug molecules.
C(3) bond of an aromatic N-heterocycle as the 2π cycloaddition partner with exclusive exo-selectivity in a highly efficient manner. It also offers a unique instance of the resulting metallocarbene species generated in this manner undergoing either oxidation by phenylmethylsulfoxide or reduction by THF, with the solvent as both the hydride and alkyl reagent source. The chemodivergent protocol exhibits exceptional functional group tolerance and is amenable to the late-stage modification of a series of structurally complex bioactive natural products and drug molecules.
中文翻译:

PtI4 催化的氧化和氢化脱芳烃 [3 + 2] 环加成 1H-吲哚 N-栓系 o-炔基苯甲醛
一种用于化学分歧组装各种高度功能化和具有结构挑战性的环庚[ b ]二氢吲哚的合成方法,它依赖于 PtI 4 -催化的氧化和氢化脱芳烃 [3 + 2] 环加成 1 H -吲哚N -栓系o -炔基苯甲醛在单个操作中进行了描述。对于在无数生物活性天然产物和药物化合物中发现的关键结构特征的合成,所提出的级联化学发散过程描绘了参与 [3 + 2] 环加成的原位形成的铂结合苯并吡喃中间体的第一个例子与 C(2)![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 芳香族 N-杂环的 C(3) 键作为 2π 环加成伙伴,以高效的方式具有独特的外向选择性。它还提供了一个独特的例子,以这种方式生成的金属碳烯物种经历了苯甲基亚砜的氧化或 THF 的还原,溶剂同时作为氢化物和烷基试剂源。chemodivergent 方案表现出卓越的官能团耐受性,并且适用于对一系列结构复杂的生物活性天然产物和药物分子进行后期修饰。
芳香族 N-杂环的 C(3) 键作为 2π 环加成伙伴,以高效的方式具有独特的外向选择性。它还提供了一个独特的例子,以这种方式生成的金属碳烯物种经历了苯甲基亚砜的氧化或 THF 的还原,溶剂同时作为氢化物和烷基试剂源。chemodivergent 方案表现出卓越的官能团耐受性,并且适用于对一系列结构复杂的生物活性天然产物和药物分子进行后期修饰。
更新日期:2022-11-14
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(3) bond of an aromatic N-heterocycle as the 2π cycloaddition partner with exclusive exo-selectivity in a highly efficient manner. It also offers a unique instance of the resulting metallocarbene species generated in this manner undergoing either oxidation by phenylmethylsulfoxide or reduction by THF, with the solvent as both the hydride and alkyl reagent source. The chemodivergent protocol exhibits exceptional functional group tolerance and is amenable to the late-stage modification of a series of structurally complex bioactive natural products and drug molecules.
C(3) bond of an aromatic N-heterocycle as the 2π cycloaddition partner with exclusive exo-selectivity in a highly efficient manner. It also offers a unique instance of the resulting metallocarbene species generated in this manner undergoing either oxidation by phenylmethylsulfoxide or reduction by THF, with the solvent as both the hydride and alkyl reagent source. The chemodivergent protocol exhibits exceptional functional group tolerance and is amenable to the late-stage modification of a series of structurally complex bioactive natural products and drug molecules.
中文翻译:

PtI4 催化的氧化和氢化脱芳烃 [3 + 2] 环加成 1H-吲哚 N-栓系 o-炔基苯甲醛
一种用于化学分歧组装各种高度功能化和具有结构挑战性的环庚[ b ]二氢吲哚的合成方法,它依赖于 PtI 4 -催化的氧化和氢化脱芳烃 [3 + 2] 环加成 1 H -吲哚N -栓系o -炔基苯甲醛在单个操作中进行了描述。对于在无数生物活性天然产物和药物化合物中发现的关键结构特征的合成,所提出的级联化学发散过程描绘了参与 [3 + 2] 环加成的原位形成的铂结合苯并吡喃中间体的第一个例子与 C(2)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 芳香族 N-杂环的 C(3) 键作为 2π 环加成伙伴,以高效的方式具有独特的外向选择性。它还提供了一个独特的例子,以这种方式生成的金属碳烯物种经历了苯甲基亚砜的氧化或 THF 的还原,溶剂同时作为氢化物和烷基试剂源。chemodivergent 方案表现出卓越的官能团耐受性,并且适用于对一系列结构复杂的生物活性天然产物和药物分子进行后期修饰。
芳香族 N-杂环的 C(3) 键作为 2π 环加成伙伴,以高效的方式具有独特的外向选择性。它还提供了一个独特的例子,以这种方式生成的金属碳烯物种经历了苯甲基亚砜的氧化或 THF 的还原,溶剂同时作为氢化物和烷基试剂源。chemodivergent 方案表现出卓越的官能团耐受性,并且适用于对一系列结构复杂的生物活性天然产物和药物分子进行后期修饰。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号