当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of the pentacyclic tetrahydroberberine pallimamine and epi-pallimamine
Tetrahedron ( IF 2.1 ) Pub Date : 2022-11-12 , DOI: 10.1016/j.tet.2022.133146 Raghavender Boda , Gregory D. Cuny
中文翻译:

五环四氢小檗碱pallimamine和epi-pallimamine的合成
更新日期:2022-11-12
Tetrahedron ( IF 2.1 ) Pub Date : 2022-11-12 , DOI: 10.1016/j.tet.2022.133146 Raghavender Boda , Gregory D. Cuny
|
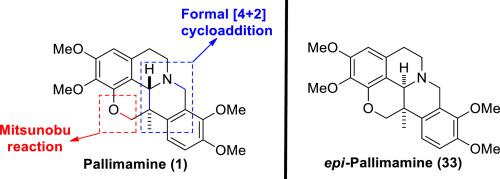
Pallimamine (1) is a structurally unique quaternary carbon-containing pentacyclic tetrahydroberberine. Synthesis of key C13-disubstituted tetrahydroberberine intermediates, as separable diastereomeric isomers, was achieved using a formal [4 + 2] cycloaddition of 4-methylhomophthalic anhydrides with 6,7-dimethoxy-3,4-dihydroisoquinolin-8-ol. Intramolecular Mitsunobu reactions provided installation of the dihydropyran generating pallimamine (1) and its non-natural diastereomer epi-pallimamine (33).
中文翻译:

五环四氢小檗碱pallimamine和epi-pallimamine的合成
Pallimamine ( 1 ) 是一种结构独特的含季碳的五环四氢小檗碱。使用 4-methylhomophthalic 酸酐与 6,7-dimethoxy-3,4-dihydroisoquinolin-8-ol 的正式 [4 + 2] 环加成反应,合成了关键的 C13-二取代四氢小檗碱中间体,作为可分离的非对映异构体。分子内 Mitsunobu 反应提供了生成二氢吡喃的 pallimamine ( 1 ) 及其非天然非对映异构体epi -pallimamine ( 33 ) 的安装。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号