当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CH3OH⋯(H2O)n [n = 1-4] clusters in external electric fields
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-05 16:03:55 , DOI: 10.1063/1.4921380
Nalini D. Gurav 1 , Anant D. Kulkarni 2 , Shridhar P. Gejji 3 , Rajeev K. Pathak 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-05 16:03:55 , DOI: 10.1063/1.4921380
Nalini D. Gurav 1 , Anant D. Kulkarni 2 , Shridhar P. Gejji 3 , Rajeev K. Pathak 1
Affiliation
|
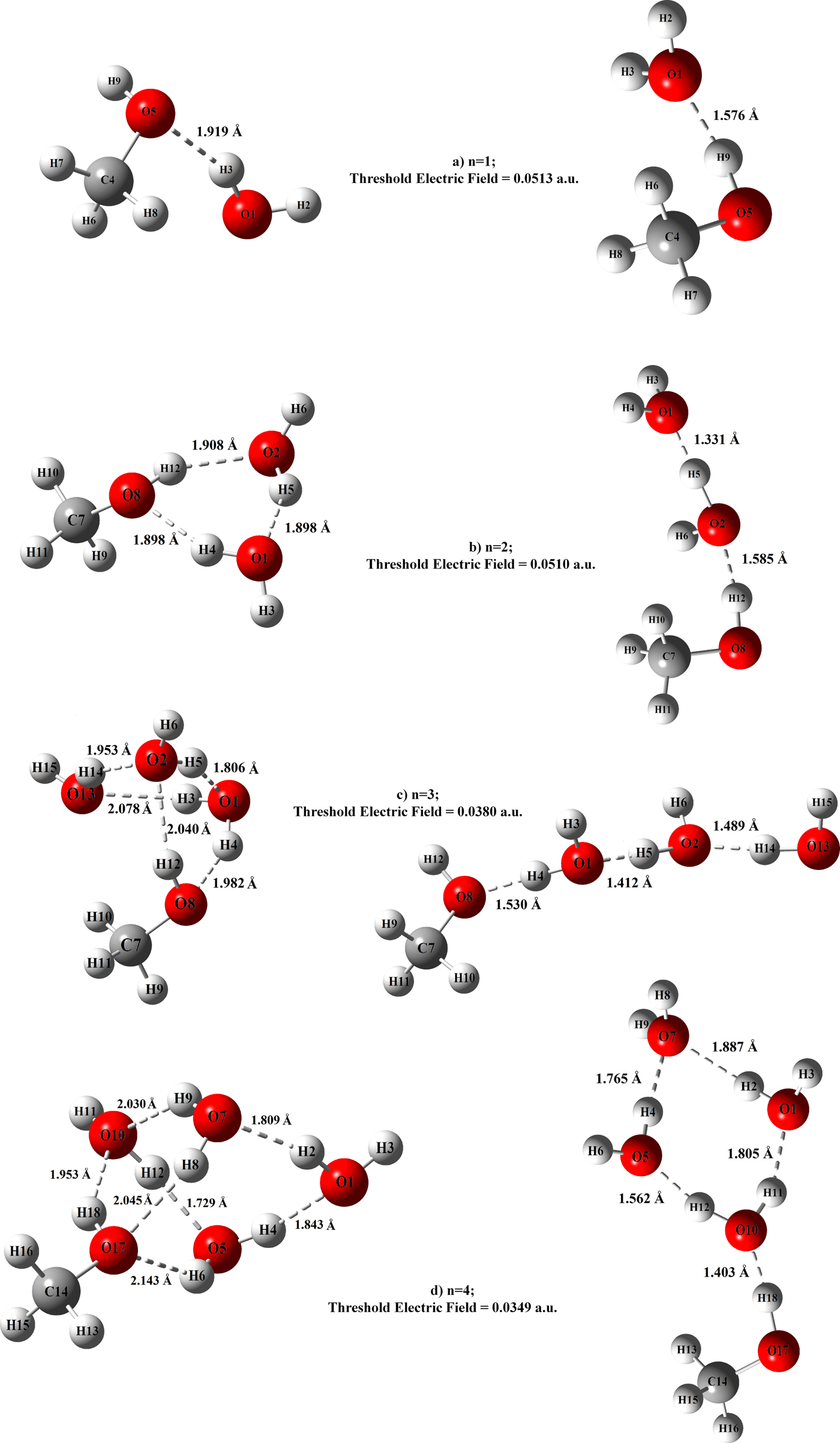
For hydrogen-bonded neutral molecular clusters, response to an externally applied electric field can critically affect molecular cooperativity. In this light, response of dilute methanol-water admixtures to an external, perturbative electric field is studied at the simplest molecular level in the cluster configurations CH3OH⋯(H2O) n with “n” chosen to range from 1 to 4, employing the M06-2X hybrid functional in conjunction with the 6-311++G(2d,2p) basis set, well-suited for hydrogen bonding. Methanol is seen to favorably bond with the water molecules at its hydroxyl end up to certain characteristic maximum threshold field strengths beyond which the HOMO-LUMO energy-gap abruptly drops to zero culminating into a complete breakdown of the cluster. In the interim regime prior to breakdown, the electric field significantly alters the hydrogen bonding pattern primarily by elongating the cluster, resulting in a marked enhancement in its electric dipole moment leading to alterations in the molecular electrostatic potential. With the application of electric field, certain “exotic” O–H vibration bands appear that at the threshold field fall in the frequency range of 2510 cm−1–1880 cm−1 in the IR spectra, in contrast with their normal (zero-field) counterparts that occur in the range of ∼3300–3900 cm−1.
中文翻译:

CH3OH⋯(H2O)n [n = 1-4]在外部电场中成簇
对于氢键键合的中性分子簇,对外部施加的电场的响应会严重影响分子的协同作用。有鉴于此,稀甲醇-水外加剂响应于外部,扰动电场进行了研究在群集配置CH最简单的分子水平3 OH⋯(H 2 O)Ñ与“ Ñ ”选择范围为1至4,采用M06-2X混合功能与6-311 ++ G(2d,2p)基组结合使用,非常适合氢键键合。可以看出甲醇在其羟基末端与水分子有利地键合,达到某些特征性的最大阈值场强,超过此阈值场强,HOMO-LUMO的能隙突然下降至零,最终使团簇完全破裂。在击穿之前的过渡状态中,电场主要通过延长团簇来显着改变氢键键合模式,从而导致其电偶极矩显着增强,从而导致分子静电势发生变化。随着电场的施加,出现了某些“奇异”的O–H振动带,它们在阈值场处落在2510 cm的频率范围内红外光谱中的-1 –1880 cm -1,与它们在约3300–3900 cm -1的范围内的正常(零场)对应物相反。
更新日期:2015-06-06
中文翻译:

CH3OH⋯(H2O)n [n = 1-4]在外部电场中成簇
对于氢键键合的中性分子簇,对外部施加的电场的响应会严重影响分子的协同作用。有鉴于此,稀甲醇-水外加剂响应于外部,扰动电场进行了研究在群集配置CH最简单的分子水平3 OH⋯(H 2 O)Ñ与“ Ñ ”选择范围为1至4,采用M06-2X混合功能与6-311 ++ G(2d,2p)基组结合使用,非常适合氢键键合。可以看出甲醇在其羟基末端与水分子有利地键合,达到某些特征性的最大阈值场强,超过此阈值场强,HOMO-LUMO的能隙突然下降至零,最终使团簇完全破裂。在击穿之前的过渡状态中,电场主要通过延长团簇来显着改变氢键键合模式,从而导致其电偶极矩显着增强,从而导致分子静电势发生变化。随着电场的施加,出现了某些“奇异”的O–H振动带,它们在阈值场处落在2510 cm的频率范围内红外光谱中的-1 –1880 cm -1,与它们在约3300–3900 cm -1的范围内的正常(零场)对应物相反。


































 京公网安备 11010802027423号
京公网安备 11010802027423号