Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-11-11 , DOI: 10.1016/j.jhazmat.2022.130386 Yuchen Zhang 1 , Peng Zhou 1 , Rongfu Huang 2 , Chenying Zhou 1 , Yang Liu 1 , Heng Zhang 1 , Xiaowei Huo 3 , Jian Zhao 4 , Zhaokun Xiong 1 , Bo Lai 1
|
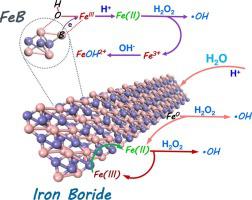
The regeneration of Fe(II) is the rate-limiting step in the Fenton/Fenton-like chain reactions that seriously hinder their scientific progress towards practical application. In this study, we proposed iron boride (FeB) for the first time as a new material to sustainably decompose H2O2 to generate hydroxyl radicals, which can non-selectively degrade a wide array of refractory organic pollutants. Fe(II) can be steadily released by the stepwise oxidation of FeB to stimulate Fenton reaction, meanwhile, B-B bonds as electron donors on the surface of FeB effectively promote the regeneration of Fe(II) from Fe(III) species and significantly accelerate the production of hydroxyl radicals. The low generation of toxic by-products and the high utilization rate of iron species validly avoid the secondary organic/metal pollution in the FeB/H2O2 system. Therefore, FeB mediated Fenton oxidation provides a novel strategy to realize a green and long-lasting environmental remediation.
中文翻译:

硼化铁促进芬顿氧化:硼物种诱导可持续的 FeIII/FeII 氧化还原对
Fe(II)的再生是Fenton/Fenton-like链式反应中的限速步骤,严重阻碍了它们在实际应用中的科学进展。在这项研究中,我们首次提出硼化铁 (FeB) 作为一种可持续分解 H 2 O 2的新材料产生羟基自由基,可以非选择性地降解多种难降解有机污染物。Fe(II)可以通过FeB的逐步氧化稳定释放出来以刺激Fenton反应,同时,FeB表面的BB键作为电子供体有效地促进了Fe(II)从Fe(III)物种的再生,显着加速了Fe(II)的生成。羟基自由基的产生。有毒副产物生成少,铁物种利用率高,有效避免了FeB/H 2 O 2体系中有机物/金属的二次污染。因此,FeB介导的芬顿氧化为实现绿色、持久的环境修复提供了一种新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号