Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-11-11 , DOI: 10.1016/j.molstruc.2022.134544 R. Arivazhagan , C. Sridevi , A. Prakasam
|
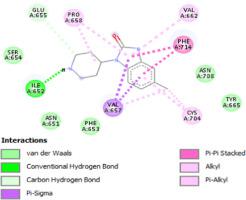
The FT-IR, FT-Raman, UV-Vis, 1H, and 13C NMR spectroscopic, molecular structure, and electronic behavior of 5-Chloro-1-(4-piperidyl)-2-benzimidazolinone (CPBI) molecule was studied in detail. The quantum computational calculations were performed by the DFT/ B3LYP method using the 6-311++G (d,p) basis set. The vibrational wavenumbers and NMR chemical shifts were calculated and compared with the experimental. A PES scan study was carried out to find the stable conformer of the CPBI molecule. The Fukui function was calculated through Mulliken population analysis (N, N+1, and N-1) to predict individual atoms' global softness and reactive sites in the CPBI molecule. MEP map reveals that the reactive sites for electrophilic and nucleophilic attack and the lower HOMO- LUMO energy gap explains that the title molecule was more reactive and least stable. The inter-and intra-molecular interactions were identified through NBO analysis, and the presence of π → π* delocalization interaction within the molecular system stabilizes the CPBI molecule. The nonlinear optical property was also calculated for the title molecule. Lipinski's rule of five and bioavailability score show that the CPBI molecule was a good candidate for protein-ligand interaction and may be suitable for the new drug design process. Finally, molecular docking studies showed that the title molecule exhibits better inhibition activity against oncology, asthma, thrombotic and cardiovascular diseases.
中文翻译:

通过光谱、分子对接和量子化学方法研究新型生物活性有机分子 5-Chloro-1-(4-piperidyl)-2-benzimidazolinone
FT-IR、FT-Raman、UV-Vis、1 H 和13详细研究了 5-Chloro-1-(4-piperidyl)-2-benzimidazolinone (CPBI) 分子的 C NMR 光谱、分子结构和电子行为。使用6-311++G(d,p)基组通过DFT/B3LYP方法进行量子计算。计算了振动波数和核磁共振化学位移,并与实验进行了比较。进行 PES 扫描研究以发现 CPBI 分子的稳定构象异构体。Fukui 函数是通过 Mulliken 布居分析(N、N+1 和 N-1)计算的,用于预测 CPBI 分子中单个原子的全局柔软度和反应位点。MEP 图揭示了亲电和亲核攻击的反应位点以及较低的 HOMO-LUMO 能隙解释了标题分子的反应性更高且最不稳定。通过 NBO 分析确定了分子间和分子内相互作用,分子系统中 π → π* 离域相互作用的存在稳定了 CPBI 分子。还计算了标题分子的非线性光学特性。Lipinski 的五规则和生物利用度得分表明 CPBI 分子是蛋白质-配体相互作用的良好候选者,可能适用于新药设计过程。最后,分子对接研究表明,标题分子对肿瘤、哮喘、血栓和心血管疾病表现出更好的抑制活性。Lipinski 的五规则和生物利用度得分表明 CPBI 分子是蛋白质-配体相互作用的良好候选者,可能适用于新药设计过程。最后,分子对接研究表明,标题分子对肿瘤、哮喘、血栓和心血管疾病表现出更好的抑制活性。Lipinski 的五规则和生物利用度得分表明 CPBI 分子是蛋白质-配体相互作用的良好候选者,可能适用于新药设计过程。最后,分子对接研究表明,标题分子对肿瘤、哮喘、血栓和心血管疾病表现出更好的抑制活性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号