Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2022-11-12 , DOI: 10.1016/j.saa.2022.122114
Khalid A M Attia 1 , Ahmed H Abdel-Monem 1 , Ahmed M Abdel-Raoof 1 , Amr S Eissa 2
|
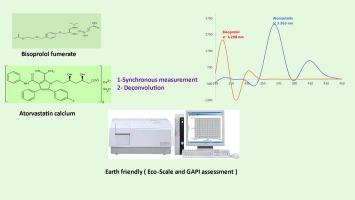
Atorvastatin and bisoprolol are two medications often prescribed together for the management of cardiovascular disease and to reduce mortality. Through a simple and direct technique based on deconvolution and synchronous of the spectrofluorometric spectra, the innovative method enables simultaneous quantification of bisoprolol and atorvastatin as single or co-formulated dosage forms in bulk and plasma. The method depends on measuring the amplitudes of bisoprolol and atorvastatin at 298 nm and 363 nm directly after deconvolution where the other drug doesn’t show interference. The linearity of the method was 0.02–0.5 µg/mL and 0.3–25 µg/mL for bisoprolol and atorvastatin, respectively. LOD and LOQ were 0.004, 0.085 µg/mL and 0.013, 0.259 µg/mL for bisoprolol and atorvastatin respectively. Furthermore, green assessment of the method using Eco-Scale and GAPI scale. The method was precise, economical, simple, smart, time-saving and eco-friendly, which allowed its application in quality control unit and in lab assessment.
中文翻译:

用于同时测定散装和血浆中比索洛尔和阿托伐他汀作为单一或共同给药药物的新型解卷积同步荧光光谱法;绿色评估
阿托伐他汀和比索洛尔是两种经常一起开处方的药物,用于治疗心血管疾病和降低死亡率。通过基于荧光光谱的解卷积和同步的简单直接技术,该创新方法能够同时定量比索洛尔和阿托伐他汀作为散装和血浆中的单一或联合制剂剂型。该方法取决于在解卷积后直接测量比索洛尔和阿托伐他汀在 298 nm 和 363 nm 的振幅,而其他药物未显示出干扰。对于比索洛尔和阿托伐他汀,该方法的线性度分别为 0.02–0.5 µg/mL 和 0.3–25 µg/mL。比索洛尔和阿托伐他汀的 LOD 和 LOQ 分别为 0.004、0.085 µg/mL 和 0.013、0.259 µg/mL。此外,绿色评价方法采用Eco-Scale和GAPI量表。该方法精确、经济、简单、智能、省时、环保,可用于质量控制单元和实验室评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号