European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-11-11 , DOI: 10.1016/j.ejmech.2022.114916 Miriam Girardini 1 , Francesca Ferlenghi 2 , Giannamaria Annunziato 1 , Giulia Degiacomi 3 , Bianca Papotti 4 , Cinzia Marchi 4 , José Camilla Sammartino 3 , Sari S Rasheed 5 , Anna Contini 1 , Maria Rosalia Pasca 3 , Federica Vacondio 2 , Joanna C Evans 6 , Thomas Dick 7 , Rolf Müller 5 , Gabriele Costantino 8 , Marco Pieroni 9
|
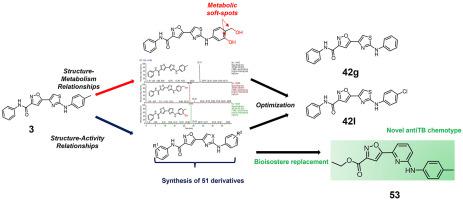
Tuberculosis is one of the deadliest infectious diseases in the world, and the increased number of multidrug-resistant and extensively drug-resistant strains is a reason for concern. We have previously reported a series of substituted 5-(2-aminothiazol-4-yl)isoxazole-3-carboxamides with growth inhibitory activity against Mycobacterium tuberculosis strains and low propensity to be substrate of efflux pumps. Encouraged by these preliminary results, we have undertaken a medicinal chemistry campaign to determine the metabolic fate of these compounds and to delineate a reliable body of Structure–Activity Relationships. Keeping intact the (thiazol-4-yl)isoxazole-3-carboxamide core, as it is deemed to be the pharmacophore of the molecule, we have extensively explored the structural modifications able to confer good activity and avoid rapid clearance. Also, a small set of analogues based on isostere manipulation of the 2-aminothiazole were prepared and tested, with the aim to disclose novel antitubercular chemotypes. These studies, combined, were instrumental in designing improved compounds such as 42g and 42l, escaping metabolic degradation by human liver microsomes and, at the same time, maintaining good antitubercular activity against both drug-susceptible and drug-resistant strains.
中文翻译:

扩展有关抗结核药物 5-(2-氨基噻唑-4-基)异恶唑-3-甲酰胺的知识:通过支架衍生化进行先导化合物优化和新型抗结核化学型的释放
结核病是世界上最致命的传染病之一,多重耐药和广泛耐药菌株数量的增加令人担忧。我们之前报道了一系列取代的 5-(2-氨基噻唑-4-基)异恶唑-3-甲酰胺,对结核分枝杆菌菌株具有生长抑制活性,并且不易成为外排泵的底物。受到这些初步结果的鼓舞,我们开展了一项药物化学活动,以确定这些化合物的代谢命运,并描绘出可靠的结构-活性关系。保持(噻唑-4-基)异恶唑-3-甲酰胺核心的完整性,因为它被认为是分子的药效团,我们广泛探索了能够赋予良好活性并避免快速清除的结构修饰。此外,还制备并测试了基于 2-氨基噻唑等配体操作的一小组类似物,目的是公开新的抗结核化学型。这些研究相结合,有助于设计改进的化合物,例如42g和42l,避免人类肝微粒体的代谢降解,同时保持针对药物敏感和耐药菌株的良好抗结核活性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号