Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-11-11 , DOI: 10.1016/j.apcatb.2022.122145 Xiya Guan , Qiannan Wu , Haibo Li , Suyuan Zeng , Qingxia Yao , Rui Li , Hongyan Chen , Yao Zheng , Konggang Qu
|
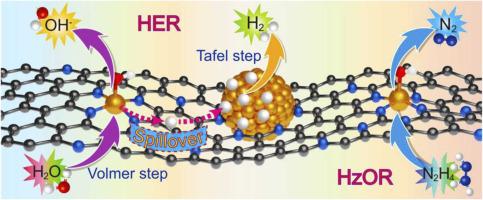
With a much lower thermodynamic reaction potential, the hydrazine oxidation reaction (HzOR) can be employed as an alternative of water oxidation reaction to integrate with the cathodic hydrogen evolution reaction (HER), accomplishing an energy-efficient H2 production. The realization of this necessitates the development of the excellent bifunctional electrocatalysts for both HER and HzOR. Herein, a common Ru complex was applied to prepare a Ru/porous N-doped carbon composite (Ru/PNC) simultaneously containing abundant Ru single atoms (SAs) and ultrafine Ru nanoclusters (1.7 nm). Firstly, the new Ru/PNC catalysts containing both metal-metal as well as metal-substrate interactions display superb HER and HzOR activities in alkaline and neutral electrolytes, both greatly surpassing the sole Ru nanoparticles or Ru SAs sample. The controlled experiments and theoretical studies unravel water dissociation and H ad-desorption occurs on Ru SAs and nanoclusters, respectively, involving the proton transfer between them during the HER process, while HzOR is mainly proceeded on Ru SAs sites. Secondly, the alkaline overall hydrazine splitting with Ru/PNC only demands a voltage of 0.19 V to achieve 100 mA cm−2, demonstrating the huge energy-saving advantage compared with conventional water splitting. Additionally, the hydrogen generation can be readily operated with the hydrazine fuel cell and commercial solar cell with the appreciable H2 production rate of 32.7 and 27.1 mL cm−2 h−1, respectively.
中文翻译:

确定 Ru 单原子和纳米团簇在电催化肼氧化辅助的节能制氢中的作用
由于热力学反应电位低得多,肼氧化反应 (HzOR) 可以作为水氧化反应的替代方案,与阴极析氢反应 (HER) 相结合,实现高效节能的 H 2生产。实现这一点需要开发用于 HER 和 HzOR 的优异双功能电催化剂。在此,应用常见的 Ru 络合物制备同时含有大量 Ru 单原子 (SAs) 和超细 Ru 纳米团簇 (1.7 nm) 的 Ru/多孔氮掺杂碳复合材料 (Ru/PNC)。首先,包含金属-金属以及金属-基底相互作用的新型 Ru/PNC 催化剂在碱性和中性电解质中表现出极好的 HER 和 HzOR 活性,两者都大大超过了单一的 Ru 纳米粒子或 Ru SAs 样品。对照实验和理论研究揭示了 Ru SAs 和纳米团簇上分别发生水离解和 H 吸附脱附,涉及在 HER 过程中它们之间的质子转移,而 HzOR 主要在 Ru SAs 位点上进行。第二,-2,与传统的水分解相比具有巨大的节能优势。此外,使用联氨燃料电池和商用太阳能电池可以很容易地产生氢气,其 H 2生产率分别为 32.7 和 27.1 mL cm -2 h -1。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号