当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Studies of 1H-Imidazo[4,5-c]quinolin-4-amine Derivatives as A3 Adenosine Receptor Positive Allosteric Modulators
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-11 , DOI: 10.1021/acs.jmedchem.2c01170 Lucas B Fallot 1, 2, 3 , R Rama Suresh 1 , Courtney L Fisher , Veronica Salmaso 1 , Robert D O'Connor 1 , Noy Kaufman 1 , Zhan-Guo Gao 1 , John A Auchampach , Kenneth A Jacobson 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-11 , DOI: 10.1021/acs.jmedchem.2c01170 Lucas B Fallot 1, 2, 3 , R Rama Suresh 1 , Courtney L Fisher , Veronica Salmaso 1 , Robert D O'Connor 1 , Noy Kaufman 1 , Zhan-Guo Gao 1 , John A Auchampach , Kenneth A Jacobson 1
Affiliation
|
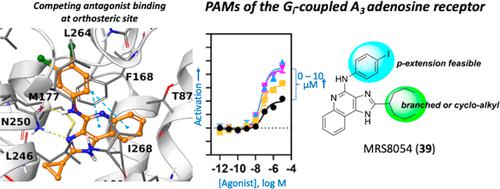
We previously reported 1H-imidazo[4,5-c]quinolin-4-amines as A3 adenosine receptor (A3AR) positive allosteric modulators (PAMs). A3AR agonists, but not PAMs, are in clinical trials for inflammatory diseases and liver conditions. We synthesized new analogues to distinguish 2-cyclopropyl antagonist 17 (orthosteric interaction demonstrated by binding and predicted computationally) from PAMs (derivatives with large 2-alkyl/cycloalkyl/bicycloalkyl groups). We predicted PAM binding at a hydrophobic site on the A3AR cytosolic interface. Although having low Caco-2 permeability and high plasma protein binding, hydrophobic 2-cyclohept-4-enyl-N-3,4-dichlorophenyl, MRS7788 18, and 2-heptan-4-yl-N-4-iodophenyl, MRS8054 39, derivatives were orally bioavailable in rat. 2-Heptan-4-yl-N-3,4-dichlorophenyl 14 and 2-cyclononyl-N-3,4-dichlorophenyl 20 derivatives and 39 greatly enhanced Cl-IB-MECA-stimulated [35S]GTPγS binding Emax, with only 12b trending toward decreasing the agonist EC50. A feasible route for radio-iodination at the p-position of a 4-phenylamino substituent suggests a potential radioligand for allosteric site binding. Herein, we advanced an allosteric approach to developing A3AR-activating drugs that are potentially event- and site-specific in action.
中文翻译:

1H-咪唑并[4,5-c]喹啉-4-胺衍生物作为 A3 腺苷受体正变构调节剂的结构-活性研究
我们之前报道了 1 H-咪唑并[4,5- c ]喹啉-4-胺作为 A 3腺苷受体 (A 3 AR) 正变构调节剂 (PAM)。A 3 AR 激动剂(而非 PAM)正在进行针对炎症性疾病和肝脏疾病的临床试验。我们合成了新的类似物来区分 2-环丙基拮抗剂17(通过结合证明的正位相互作用并通过计算预测)与 PAM(具有大的 2-烷基/环烷基/双环烷基基团的衍生物)。我们预测 PAM 在 A 3 AR 胞质界面上的疏水位点结合。尽管具有低 Caco-2 渗透性和高血浆蛋白结合性,但疏水性 2-环庚-4-烯基-N -3,4-二氯苯基,MRS7788 18和 2-庚烷-4-基-N -4-碘苯基,MRS8054 39,衍生物在大鼠中具有口服生物利用度。2-庚烷-4-基-N -3,4-二氯苯基14和2-环壬基-N -3,4-二氯苯基20种衍生物和39种大大增强了Cl-IB-MECA刺激的[ 35S ]GTPγS结合E max,只有12b有降低激动剂 EC 50的趋势。在4-苯氨基取代基的对位进行放射性碘化的可行途径表明了用于变构位点结合的潜在放射性配体。在此,我们提出了一种变构方法来开发 A 3 AR 激活药物,这些药物具有潜在的事件和位点特异性作用。
更新日期:2022-11-11
中文翻译:

1H-咪唑并[4,5-c]喹啉-4-胺衍生物作为 A3 腺苷受体正变构调节剂的结构-活性研究
我们之前报道了 1 H-咪唑并[4,5- c ]喹啉-4-胺作为 A 3腺苷受体 (A 3 AR) 正变构调节剂 (PAM)。A 3 AR 激动剂(而非 PAM)正在进行针对炎症性疾病和肝脏疾病的临床试验。我们合成了新的类似物来区分 2-环丙基拮抗剂17(通过结合证明的正位相互作用并通过计算预测)与 PAM(具有大的 2-烷基/环烷基/双环烷基基团的衍生物)。我们预测 PAM 在 A 3 AR 胞质界面上的疏水位点结合。尽管具有低 Caco-2 渗透性和高血浆蛋白结合性,但疏水性 2-环庚-4-烯基-N -3,4-二氯苯基,MRS7788 18和 2-庚烷-4-基-N -4-碘苯基,MRS8054 39,衍生物在大鼠中具有口服生物利用度。2-庚烷-4-基-N -3,4-二氯苯基14和2-环壬基-N -3,4-二氯苯基20种衍生物和39种大大增强了Cl-IB-MECA刺激的[ 35S ]GTPγS结合E max,只有12b有降低激动剂 EC 50的趋势。在4-苯氨基取代基的对位进行放射性碘化的可行途径表明了用于变构位点结合的潜在放射性配体。在此,我们提出了一种变构方法来开发 A 3 AR 激活药物,这些药物具有潜在的事件和位点特异性作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号