当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Condensations with biaryl ethers and carbonyl groups leading to spirocycles
Tetrahedron ( IF 2.1 ) Pub Date : 2022-11-10 , DOI: 10.1016/j.tet.2022.133123 Jeffrey C. Ferreira , Jacob C. Hood , Douglas A. Klumpp
中文翻译:

与联芳基醚和羰基缩合形成螺环
更新日期:2022-11-10
Tetrahedron ( IF 2.1 ) Pub Date : 2022-11-10 , DOI: 10.1016/j.tet.2022.133123 Jeffrey C. Ferreira , Jacob C. Hood , Douglas A. Klumpp
|
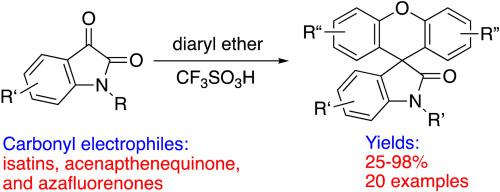
Biaryl ethers condense directly with carbonyl groups on diazafluorenones, acenaphthenequinone, and isatins, to give a variety of spirocyclic compounds. The condensation reactions are promoted by the Brønsted superacid, CF3SO3H (triflic acid). This methodology provides a convenient one-pot synthetic route to aromatic spirocycles.
中文翻译:

与联芳基醚和羰基缩合形成螺环
联芳基醚直接与二氮杂芴酮、苊醌和靛红上的羰基缩合,得到多种螺环化合物。Brønsted 超强酸 CF 3 SO 3 H(三氟甲磺酸)可促进缩合反应。该方法为芳香螺环化合物提供了一条方便的一锅法合成路线。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号