Water Research ( IF 11.4 ) Pub Date : 2022-11-08 , DOI: 10.1016/j.watres.2022.119346
Fei Miao 1 , Xiting Yue 1 , Cheng Cheng 1 , Xuantong Chen 1 , Wei Ren 2 , Hui Zhang 1
|
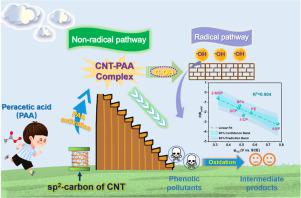
Peracetic-acid-based advanced oxidation processes (PAA-AOPs) on metal-free catalysts have emerged as charming strategies for water contaminant removal. However, the involved reactive species and their corresponding active sites are ambiguous. Herein, using carbon nanotube (CNT) as a model carbocatalyst, we demonstrated that, under neutral conditions, the CNT−PAA* complex was the dominant reactive species to oxidize phenolic compounds via electron-transfer process (ETP), whereas the surface-bound hydroxyl radicals (·OHsurface) played a minor role on the basis of quenching and electrochemical tests as well as Raman spectroscopy. More importantly, the experimental and density functional theory (DFT) calculation results collaboratively proved that the active site for ETP was the sp2-hybridized carbon on the CNT bulk, while that for radical generation was the edge-located hydroxyl group (C−OH), which lowered the energy barrier for cleaving the O−O bond in CNT−PAA* complex. We further discerned the oxidation kinetic constants (koxid) of different pollutants from the apparent kinetic constants in CNT/PAA system. The significant negative linear correlation between lnkoxid and half-wave potential of phenolic compounds suggests that the pollutants with a lower one-electron oxidation potential (i.e., stronger electron-donating ability) are more easily oxidized. Overall, this study scrutinizes the hybrid radical and non-radical mechanism and the corresponding active sites of the CNT/PAA system, providing insights into the application of PAA-AOPs and the development of ETP in the remediation of emerging organic pollutants.
中文翻译:

深入了解过乙酸活化的碳催化机制:动力学识别和活性位点识别
基于无金属催化剂的基于过氧乙酸的高级氧化工艺 (PAA-AOP) 已成为去除水污染物的迷人策略。然而,所涉及的活性物质及其相应的活性位点是不明确的。在此,我们使用碳纳米管 (CNT) 作为模型碳催化剂,证明了在中性条件下,CNT-PAA* 复合物是通过电子转移过程 (ETP) 氧化酚类化合物的主要活性物质,而表面结合的根据猝灭和电化学测试以及拉曼光谱,羟基自由基(· OH表面)发挥了次要作用。更重要的是,实验和密度泛函理论(DFT)计算结果共同证明了ETP的活性位点是sp2-杂化碳在 CNT 本体上,而产生自由基的是位于边缘的羟基 (C-OH),这降低了 CNT-PAA* 复合物中 O-O 键断裂的能垒。我们进一步从 CNT/PAA 系统的表观动力学常数中辨别出不同污染物的氧化动力学常数 ( k oxid )。ln k oxid之间显着负线性相关酚类化合物的半波电位表明单电子氧化电位越低(即给电子能力越强)的污染物越容易被氧化。总的来说,本研究仔细研究了 CNT/PAA 体系的杂化自由基和非自由基机制以及相应的活性位点,为 PAA-AOPs 的应用和 ETP 在新兴有机污染物修复中的发展提供了见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号