Cell ( IF 45.5 ) Pub Date : 2022-11-10 , DOI: 10.1016/j.cell.2022.10.018 John Janetzko 1 , Ryoji Kise 2 , Benjamin Barsi-Rhyne 3 , Dirk H Siepe 4 , Franziska M Heydenreich 1 , Kouki Kawakami 2 , Matthieu Masureel 1 , Shoji Maeda 1 , K Christopher Garcia 4 , Mark von Zastrow 3 , Asuka Inoue 2 , Brian K Kobilka 1
|
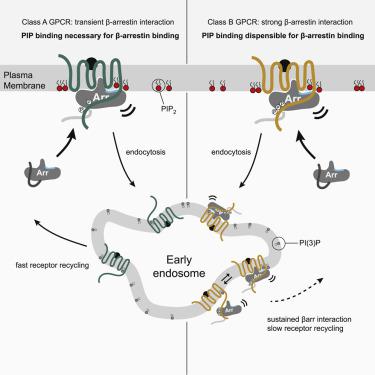
Binding of arrestin to phosphorylated G protein-coupled receptors (GPCRs) is crucial for modulating signaling. Once internalized, some GPCRs remain complexed with β-arrestins, while others interact only transiently; this difference affects GPCR signaling and recycling. Cell-based and in vitro biophysical assays reveal the role of membrane phosphoinositides (PIPs) in β-arrestin recruitment and GPCR-β-arrestin complex dynamics. We find that GPCRs broadly stratify into two groups, one that requires PIP binding for β-arrestin recruitment and one that does not. Plasma membrane PIPs potentiate an active conformation of β-arrestin and stabilize GPCR-β-arrestin complexes by promoting a fully engaged state of the complex. As allosteric modulators of GPCR-β-arrestin complex dynamics, membrane PIPs allow for additional conformational diversity beyond that imposed by GPCR phosphorylation alone. For GPCRs that require membrane PIP binding for β-arrestin recruitment, this provides a mechanism for β-arrestin release upon translocation of the GPCR to endosomes, allowing for its rapid recycling.
中文翻译:

膜磷酸肌醇调节 GPCR-β-抑制蛋白复合物的组装和动力学
视紫红质抑制蛋白与磷酸化 G 蛋白偶联受体 (GPCR) 的结合对于调节信号传导至关重要。一旦内化,一些 GPCR 仍与 β-arrestins 复合,而其他 GPCR 仅短暂相互作用;这种差异会影响 GPCR 信号传导和回收。基于细胞和体外生物物理测定揭示了膜磷酸肌醇 (PIP) 在 β-抑制蛋白募集和 GPCR-β-抑制蛋白复合物动力学中的作用。我们发现 GPCR 大致分为两类,一组需要 PIP 结合来招募 β-arrestin,另一组则不需要。质膜 PIP 增强 β-arrestin 的活性构象,并通过促进复合物的完全接合状态来稳定 GPCR-β-arrestin 复合物。作为 GPCR-β-arrestin 复合物动力学的变构调节剂,膜 PIP 可以实现除 GPCR 磷酸化单独施加的构象多样性之外的额外构象多样性。对于需要膜 PIP 结合来募集 β-抑制蛋白的 GPCR,这提供了一种在 GPCR 易位至内体时释放 β-抑制蛋白的机制,从而使其能够快速回收。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号