当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Ammonia Oxidation by a Low-Coordinate Copper Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-09 , DOI: 10.1021/jacs.2c07977 Md Estak Ahmed 1, 2 , Mahdi Raghibi Boroujeni 2 , Pokhraj Ghosh 1, 2 , Christine Greene 2 , Subrata Kundu 2, 3 , Jeffery A Bertke 2 , Timothy H Warren 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-09 , DOI: 10.1021/jacs.2c07977 Md Estak Ahmed 1, 2 , Mahdi Raghibi Boroujeni 2 , Pokhraj Ghosh 1, 2 , Christine Greene 2 , Subrata Kundu 2, 3 , Jeffery A Bertke 2 , Timothy H Warren 1, 2
Affiliation
|
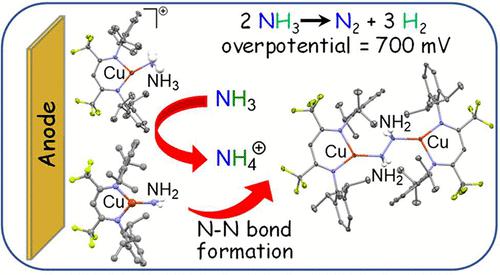
Molecular catalysts for ammonia oxidation to dinitrogen represent enabling components to utilize ammonia as a fuel and/or source of hydrogen. Ammonia oxidation requires not only the breaking of multiple strong N–H bonds but also controlled N–N bond formation. We report a novel β-diketiminato copper complex [iPr2NNF6]CuI-NH3 ([CuI]-NH3 (2)) as a robust electrocatalyst for NH3 oxidation in acetonitrile under homogeneous conditions. Complex 2 operates at a moderate overpotential (η = 700 mV) with a TOFmax = 940 h–1 as determined from CV data in 1.3 M NH3–MeCN solvent. Prolonged (>5 h) controlled potential electrolysis (CPE) reveals the stability and robustness of the catalyst under electrocatalytic conditions. Detailed mechanistic investigations indicate that electrochemical oxidation of [CuI]-NH3 forms {[CuII]-NH3}+ (4), which undergoes deprotonation by excess NH3 to form reactive copper(II)–amide ([CuII]-NH2, 6) unstable toward N–N bond formation to give the dinuclear hydrazine complex [CuI]2(μ-N2H4). Electrochemical studies reveal that the diammine complex [CuI](NH3)2 (7) forms at high ammonia concentration as part of the {[CuII](NH3)2}+/[CuI](NH3)2 redox couple that is electrocatalytically inactive. DFT analysis reveals a much higher thermodynamic barrier for deprotonation of the four-coordinate {[CuII](NH3)2}+ (8) by NH3 to give the copper(II) amide [CuII](NH2)(NH3) (9) (ΔG = 31.7 kcal/mol) as compared to deprotonation of the three-coordinate {[CuII]-NH3}+ by NH3 to provide the reactive three-coordinate parent amide [CuII]-NH2 (ΔG = 18.1 kcal/mol) susceptible to N–N coupling to form [CuI]2(μ-N2H4) (ΔG = −11.8 kcal/mol).
中文翻译:

低配位铜配合物电催化氨氧化
用于将氨氧化为二氮的分子催化剂代表能够利用氨作为燃料和/或氢源的组分。氨氧化不仅需要断裂多个强 N-H 键,还需要控制 N-N 键的形成。我们报告了一种新型 β-二酮亚基铜配合物 [ i Pr 2 NN F6 ]Cu I -NH 3 ([Cu I ]-NH 3 ( 2 )) 作为均相条件下乙腈中 NH 3氧化的稳健电催化剂。复合物2在中等过电位 (η = 700 mV) 下运行,TOF max = 940 h –1根据 1.3 M NH 3 –MeCN 溶剂中的 CV 数据确定。长时间(>5 小时)的受控电位电解 (CPE) 揭示了催化剂在电催化条件下的稳定性和稳健性。详细的机理研究表明,[Cu I ]-NH 3的电化学氧化形成 {[Cu II ]-NH 3 } + ( 4 ),后者会被过量的 NH 3去质子化,形成活性铜 (II)–酰胺 ([Cu II ]-NH 3 } + ( 4 ) ]-NH 2 , 6 ) 对 N-N 键形成不稳定,得到双核肼络合物 [Cu I ] 2 (μ-N 24 )。电化学研究表明,二氨络合物 [Cu I ](NH 3 ) 2 ( 7 ) 在高氨浓度下作为 {[Cu II ](NH 3 ) 2 } + /[Cu I ](NH 3 ) 2的一部分形成电催化失活的氧化还原对。DFT 分析揭示了四配位 {[Cu II ](NH 3 ) 2 } + ( 8 ) 被 NH 3去质子化得到氨基铜 (II) [CuII ](NH 2 )(NH 3 ) ( 9 ) (Δ G = 31.7 kcal/mol) 与三配位 {[Cu II ]-NH 3 } +被 NH 3去质子化以提供反应性三-相比配位母体酰胺 [Cu II ]-NH 2 (Δ G = 18.1 kcal/mol) 易受 N–N 偶联形成 [Cu I ] 2 (μ-N 2 H 4 ) (Δ G = −11.8 kcal/mol) .
更新日期:2022-11-09
中文翻译:

低配位铜配合物电催化氨氧化
用于将氨氧化为二氮的分子催化剂代表能够利用氨作为燃料和/或氢源的组分。氨氧化不仅需要断裂多个强 N-H 键,还需要控制 N-N 键的形成。我们报告了一种新型 β-二酮亚基铜配合物 [ i Pr 2 NN F6 ]Cu I -NH 3 ([Cu I ]-NH 3 ( 2 )) 作为均相条件下乙腈中 NH 3氧化的稳健电催化剂。复合物2在中等过电位 (η = 700 mV) 下运行,TOF max = 940 h –1根据 1.3 M NH 3 –MeCN 溶剂中的 CV 数据确定。长时间(>5 小时)的受控电位电解 (CPE) 揭示了催化剂在电催化条件下的稳定性和稳健性。详细的机理研究表明,[Cu I ]-NH 3的电化学氧化形成 {[Cu II ]-NH 3 } + ( 4 ),后者会被过量的 NH 3去质子化,形成活性铜 (II)–酰胺 ([Cu II ]-NH 3 } + ( 4 ) ]-NH 2 , 6 ) 对 N-N 键形成不稳定,得到双核肼络合物 [Cu I ] 2 (μ-N 24 )。电化学研究表明,二氨络合物 [Cu I ](NH 3 ) 2 ( 7 ) 在高氨浓度下作为 {[Cu II ](NH 3 ) 2 } + /[Cu I ](NH 3 ) 2的一部分形成电催化失活的氧化还原对。DFT 分析揭示了四配位 {[Cu II ](NH 3 ) 2 } + ( 8 ) 被 NH 3去质子化得到氨基铜 (II) [CuII ](NH 2 )(NH 3 ) ( 9 ) (Δ G = 31.7 kcal/mol) 与三配位 {[Cu II ]-NH 3 } +被 NH 3去质子化以提供反应性三-相比配位母体酰胺 [Cu II ]-NH 2 (Δ G = 18.1 kcal/mol) 易受 N–N 偶联形成 [Cu I ] 2 (μ-N 2 H 4 ) (Δ G = −11.8 kcal/mol) .




















































 京公网安备 11010802027423号
京公网安备 11010802027423号