当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of cobalt(II) phenolate selenoether complexes to mimic hydrogenase-like activity for hydrogen gas production
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2dt02820d Aditya Upadhyay 1 , Kanika 1 , Yogesh Mandhar 1 , Monojit Batabyal 1 , Saravanan Raju 1 , Svastik Jaiswal 1 , Ray J Butcher 2 , Sangit Kumar 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2dt02820d Aditya Upadhyay 1 , Kanika 1 , Yogesh Mandhar 1 , Monojit Batabyal 1 , Saravanan Raju 1 , Svastik Jaiswal 1 , Ray J Butcher 2 , Sangit Kumar 1
Affiliation
|
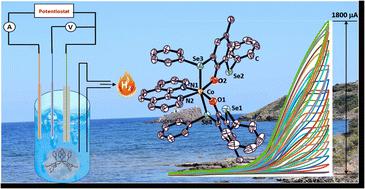
Selenium-derived electrocatalysts have been well explored for electrocatalytic hydrogen evolution reactions to mimic hydrogenase-like activity; however, the stability of these synthetic mimics is yet to be enhanced. In this study, we report the synthesis and characterization of a series of 1,10-phenanthroline-cobalt(II) phenolate selenoether complexes using 1,10-phenanthroline and 6-nitro-1,10-phenanthroline-Co(II)-dichloride and substituted bis-selenophenolate ligands. The synthesized cobalt(II) phenolate selenoether complexes have been characterized by CHN analysis, mass spectrometry, single crystal XRD, and UV-visible absorption spectroscopy. These complexes show electrocatalytic proton reduction from acetic acid at an overpotential of 0.45–0.56 V vs. Fc+/Fc and surpass previously reported selenium and sulfur-containing electrocatalysts. Furthermore, gas analysis from control potential electrolysis confirms that the cobalt(II) selenoethers act as electrocatalysts to produce H2 with a faradaic efficiency of 43–83% and show a turnover number of 3.24–58.60 molcat−1. The hydrogen evolution reaction (HER) was probed using deuterated acetic acid, which demonstrates an inverse kinetic isotopic effect (KIE) and is consistent with the formation of metal hydride intermediates. Furthermore, control experiments (post-dip analysis and multiple CV studies) have been performed to support the catalysis being due to a homogeneous process. Acid titration using UV-visible spectroscopy reveals that protonation is the prior step for electrocatalysis and assists in the formation of a cobalt hydride intermediate, which upon reaction with a proton generates hydrogen gas.
中文翻译:

钴(II)酚盐硒醚配合物的合成以模拟氢气生产的氢化酶样活性
硒衍生的电催化剂已被很好地探索用于电催化析氢反应以模拟氢化酶样活性;然而,这些合成模拟物的稳定性还有待提高。在本研究中,我们报道了使用 1,10-菲咯啉和 6-硝基-1,10-菲咯啉-Co( II )-二氯化物合成和表征一系列 1,10-菲咯啉-钴 ( II ) 酚盐硒醚络合物和取代的双硒酚配体。已通过 CHN 分析、质谱、单晶 XRD 和紫外-可见吸收光谱对合成的钴 ( II ) 酚盐硒醚配合物进行了表征。这些配合物显示出在 0.45–0.56 V 的过电位下从乙酸电催化质子还原与Fc + /Fc 相比,优于先前报道的含硒和含硫电催化剂。此外,来自控制电势电解的气体分析证实,钴 ( II ) 硒醚作为电催化剂产生 H 2,法拉第效率为 43–83%,转换数为 3.24–58.60 mol cat -1. 使用氘化乙酸探测析氢反应 (HER),证明了逆动力学同位素效应 (KIE),并且与金属氢化物中间体的形成一致。此外,已经进行了对照实验(浸后分析和多个 CV 研究)以支持催化作用是由于均相过程引起的。使用紫外-可见光谱进行酸滴定表明,质子化是电催化的前一步,有助于形成钴氢化物中间体,后者与质子反应后会产生氢气。
更新日期:2022-11-09
中文翻译:

钴(II)酚盐硒醚配合物的合成以模拟氢气生产的氢化酶样活性
硒衍生的电催化剂已被很好地探索用于电催化析氢反应以模拟氢化酶样活性;然而,这些合成模拟物的稳定性还有待提高。在本研究中,我们报道了使用 1,10-菲咯啉和 6-硝基-1,10-菲咯啉-Co( II )-二氯化物合成和表征一系列 1,10-菲咯啉-钴 ( II ) 酚盐硒醚络合物和取代的双硒酚配体。已通过 CHN 分析、质谱、单晶 XRD 和紫外-可见吸收光谱对合成的钴 ( II ) 酚盐硒醚配合物进行了表征。这些配合物显示出在 0.45–0.56 V 的过电位下从乙酸电催化质子还原与Fc + /Fc 相比,优于先前报道的含硒和含硫电催化剂。此外,来自控制电势电解的气体分析证实,钴 ( II ) 硒醚作为电催化剂产生 H 2,法拉第效率为 43–83%,转换数为 3.24–58.60 mol cat -1. 使用氘化乙酸探测析氢反应 (HER),证明了逆动力学同位素效应 (KIE),并且与金属氢化物中间体的形成一致。此外,已经进行了对照实验(浸后分析和多个 CV 研究)以支持催化作用是由于均相过程引起的。使用紫外-可见光谱进行酸滴定表明,质子化是电催化的前一步,有助于形成钴氢化物中间体,后者与质子反应后会产生氢气。













































 京公网安备 11010802027423号
京公网安备 11010802027423号