Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-11-09 , DOI: 10.1016/j.bmcl.2022.129061 Sung-Hwa Yoon 1 , Duk-Yeon Cho 2 , Jun-Hyuk Han 2 , Dong-Kug Choi 2 , Eunha Kim 1 , Ju-Young Park 3
|
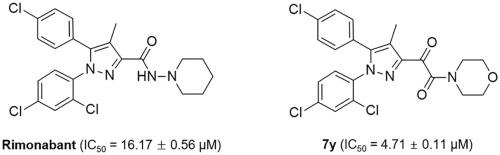
A series of rimonabant analogues, where the N-aminopiperidine moiety was replaced by various amines and an additional carbonyl group, were synthesized and their inhibition of nitric oxide (NO) production was evaluated in lipopolysaccharide (LPS)-induced BV2 microglial cells. Among the synthesized compounds, the morpholine analogue 7y (IC50 = 4.71 ± 0.11 μM) showed significantly higher inhibitory activity than rimonabant (IC50 = 16.17 ± 0.56 μM), and suppressed NO production dose-dependently without cytotoxicity. In addition, 7y inhibited the expression of iNOS, COX-2 and pro-inflammatory cytokines and attenuated LPS-induced activation of nuclear factor-kappa B (NF-κB) and ERK MAPK phosphorylation in BV2 cells. These results demonstrated that 7y exerted anti-inflammatory effects by ERK pathway in BV2 cells, which can be used for the prevention and treatment of neuroinflammatory diseases.
中文翻译:

1-(5-(4-氯苯基)-1-(2,4-二氯苯基)-4-甲基-1H-吡唑-3-基)-2-吗啉代乙烷-1,2-二酮类似物的合成及其抑制活性在脂多糖诱导的 BV2 细胞中具有降低的细胞毒性
合成了一系列利莫那班类似物,其中N-氨基哌啶部分被各种胺和一个额外的羰基取代,并在脂多糖 (LPS) 诱导的 BV2 小胶质细胞中评估了它们对一氧化氮 (NO) 产生的抑制作用。在合成的化合物中,吗啉类似物7y (IC 50 = 4.71 ± 0.11 μM) 显示出比利莫那班 (IC 50 = 16.17 ± 0.56 μM) 显着更高的抑制活性 ,并且剂量依赖性地抑制 NO 产生而没有细胞毒性。此外,7y抑制 BV2 细胞中 iNOS、COX-2 和促炎细胞因子的表达,并减弱 LPS 诱导的核因子-κB (NF-κB) 和 ERK MAPK 磷酸化的激活。这些结果表明7y通过ERK途径在BV2细胞中发挥抗炎作用,可用于神经炎症性疾病的预防和治疗。































 京公网安备 11010802027423号
京公网安备 11010802027423号