European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-11-08 , DOI: 10.1016/j.ejmech.2022.114903
Tong Qin 1 , Xuefeng Gao 2 , Lei Lei 2 , Wenxuan Zhang 1 , Jing Feng 1 , Xing Wang 3 , Zhufang Shen 2 , Zhenming Liu 4 , Yi Huan 2 , Song Wu 1 , Jie Xia 1 , Liangren Zhang 4
|
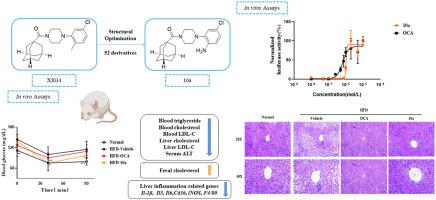
Farnesoid X receptor (FXR) is an attractive target for drug discovery against non-alcoholic fatty liver disease (NAFLD). We previously reported an orally active, new-chemotype FXR agonist XJ034 by ensemble learning-driven drug discovery. However, its FXR agonistic activity and the efficacy in vivo remain to be improved. In this study, we designed and synthesized 52 derivatives, and preliminarily evaluated their FXR transactivation activity in HEK293T cells at the concentration of 10 μM. 12 FXR agonists were superior or comparable to compound XJ034, two of which showed over 9-fold activity of compound XJ034, and were as potent as OCA. The molecular docking and molecular dynamics simulations implied an additional hydrogen bond with TYR383 is involved in FXR transactivation for both compounds. According to EC50 determined by the confirmatory transactivation assay, we selected adamantan-1-yl(4-(2-amino-5-chlorophenyl)piperazin-1-yl)methanone (10a, EC50: 1.05 μM) as our lead compound. Interestingly, compound 10a had no agonistic effect on TGR5 or PPAR, and no cytotoxicity to HepG2 cells. In vivo bioassays with high-fat-diet induced C57BL/6J obese (DIO) mice have shown that compound 10a (100 mg/kg) is more effective than compound XJ034 (200 mg/kg) in improving hyperlipidemia, hepatic steatosis and insulin resistance. We also observed that compound 10a down-regulated the expression of genes involved in liver inflammation in vivo, implying its potential to treat hepatic inflammation. In summary, the present data have proved that our strategy for structural optimization is effective, and compound 10a is a promising lead compound with improved efficacy for NAFLD.
中文翻译:

1-金刚烷基羰基-4-苯基哌嗪衍生物作为 NAFLD FXR 激动剂的结构优化和生物学评价
法尼醇 X 受体 (FXR) 是针对非酒精性脂肪性肝病 (NAFLD) 的药物发现的一个有吸引力的目标。我们之前通过集成学习驱动的药物发现报告了一种具有口服活性的新化学型 FXR 激动剂XJ034。然而,其 FXR 激动活性和体内疗效仍有待提高。在本研究中,我们设计并合成了52个衍生物,并初步评估了它们在10 μM浓度下在HEK293T细胞中的FXR反式激活活性。12 种 FXR 激动剂优于或相当于化合物XJ034,其中两种表现出化合物 XJ034 的 9 倍以上的活性,和 OCA 一样有效。分子对接和分子动力学模拟暗示与 TYR383 的额外氢键参与两种化合物的 FXR 反式激活。根据验证性反式激活试验确定的 EC 50,我们选择金刚烷-1-基(4-(2-氨基-5-氯苯基)哌嗪-1-基)甲酮 ( 10a, EC 50 : 1.05 μM )作为我们的先导化合物. 有趣的是,化合物10a对 TGR5 或 PPAR 没有激动作用,对 HepG2 细胞也没有细胞毒性。高脂肪饮食诱导的 C57BL/6J 肥胖 (DIO) 小鼠体内生物测定表明化合物10a (100 mg/kg) 比化合物XJ034更有效(200 mg/kg) 改善高脂血症、肝脂肪变性和胰岛素抵抗。我们还观察到化合物10a在体内下调了与肝脏炎症有关的基因的表达,这意味着它具有治疗肝脏炎症的潜力。总之,目前的数据已经证明我们的结构优化策略是有效的,化合物10a是一种很有前途的先导化合物,可以提高 NAFLD 的疗效。































 京公网安备 11010802027423号
京公网安备 11010802027423号